8.E: Nadharia za Juu za Bonding Covalent (Mazoezi)
- Page ID
- 175952
8.1: Theory ya dhamana ya Valence
Sura ya Mazoezi
- Eleza jinsi vifungo σ na π vinavyolingana na jinsi vinavyo tofauti.
- Chora safu inayoelezea nishati ya mfumo na atomi za H na Cl kwa umbali tofauti. Kisha, tafuta nishati ya chini ya njia hii mbili.
- Tumia nishati ya dhamana iliyopatikana katika Jedwali 8.2.1 ili kuhesabu nishati kwa dhamana moja ya HCl (Kidokezo: Ni vifungo vingi vilivyo kwenye mole?)
- Tumia enthalpy ya majibu na nguvu za dhamana\(H_2\) na\(Cl_2\) kutatua kwa nishati ya mole moja ya vifungo vya HCl. \[H_{2(g)}+Cl_{2(g)} \rightleftharpoons 2HCl_{(g)} \;\;\; ΔH^∘_{rxn}=−184.7\; kJ/mol\]
- Eleza kwa nini vifungo hutokea kwa umbali maalum wa dhamana ya wastani badala ya atomi zinazokaribia karibu sana.
- Matumizi valence dhamana nadharia kueleza bonding katika F 2, HF, na ClBr. Mchoro mwingiliano wa orbitals atomiki kushiriki katika vifungo.
- Matumizi valence dhamana nadharia kueleza bonding katika O 2. Mchoro mwingiliano wa orbitals atomiki kushiriki katika vifungo katika O 2.
- Ni vifungo ngapi vya σ na π vilivyopo katika HCN ya molekuli?
- Rafiki anakuambia N 2 ina vifungo vitatu π kutokana na mwingiliano wa tatu p -orbitals kwenye kila atomu N. Je, unakubaliana?
- Chora miundo ya Lewis kwa CO 2 na CO, na utabiri idadi ya σ na π vifungo kwa kila molekuli.
- CO 2
- USHIRIKIANO
Solutions
1. Kufanana: Aina zote mbili za vifungo zinatokana na kuingiliana kwa orbitals atomiki kwenye atomi zilizo karibu na zina kiwango cha juu cha elektroni mbili. Tofauti: Vifungo σ vina nguvu na vinatokana na mwingiliano wa mwisho hadi mwisho na vifungo vyote vya moja ni vifungo σ; π vifungo kati ya atomi hizo mbili ni dhaifu kwa sababu zinatokana na kuingiliana kwa upande mmoja, na vifungo vingi vina vifungo moja au zaidi π (pamoja na dhamana ya σ).
2
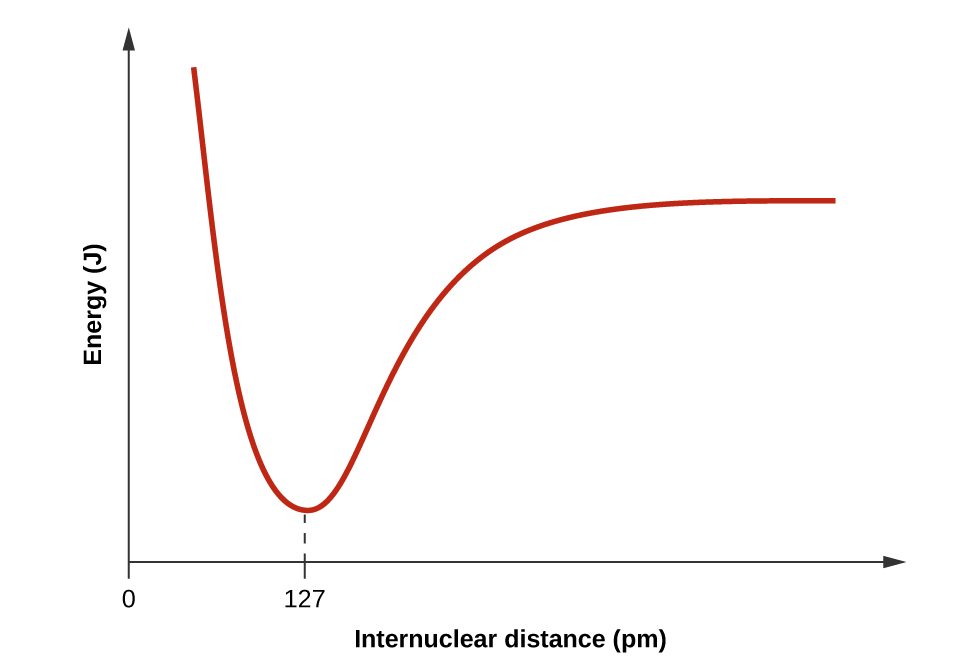
Wakati H na Cl ni tofauti (mhimili x) nishati ni kwa thamani fulani. Wanapokaribia, hupungua kwa kiwango cha chini saa 127 jioni (umbali wa dhamana), na kisha huongezeka kwa kasi unapofika karibu.
- (a) H—Cl431 KJ/mol 427KJMOL×Mol6.022×1023bonds×1000 JKJ = 7.09×10-19
- (b) Tunajua sheria ya Hess kuhusiana na nguvu za dhamana: ΔH° =ΔHBDE (kuvunjwa) ΔHBDE (sumu) Tunapewa enthalpy ya mmenyuko
\[−184.7 kJ/mol=(ΔH∘BDE(H–H)+ΔH∘BDE(Cl–Cl))−(2ΔH∘BDE(H–Cl))\]
\[H–H is 436 kJ/mol and Cl–Cl is 243\]
\[–184.7 kJ/mol = (436 + 243) – 2x = 679 – 2x\]
\[2x = 863.7 kJ/mol\]
\[x = 432\; kJ/mol\]
Hii ni karibu sana na thamani kutoka sehemu (a).
3. Umbali wa dhamana ya wastani ni umbali na nishati ya chini kabisa. Katika umbali chini ya umbali wa dhamana, mashtaka mazuri kwenye nuclei mbili hurudiana, na nishati ya jumla huongezeka.
4. Dhamana moja iliyopo katika kila molekuli husababishwa na kuingiliana kwa orbitals husika: F 2 p orbitals katika F 2, H 1 s na F 2 p orbitals katika HF, na Cl 3 p orbital na Br 4 p orbital katika ClBr.
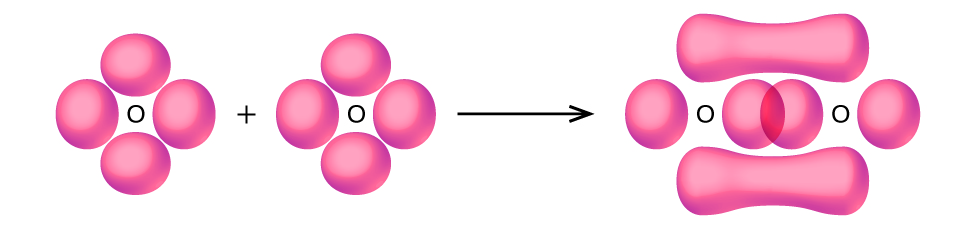
5. Bonding: Moja σ dhamana na moja π dhamana. Orbitals s ni kujazwa na wala kuingiliana. Orbitali p huingiliana kando ya mhimili ili kuunda dhamana ya σ na upande kwa upande ili kuunda dhamana π.
6. \(\ce{H–C≡N}\)ina mbili σ (H—C na C—N) na mbili π (kufanya dhamana tatu ya CN).
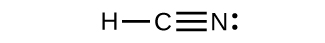
7. Hapana, mbili za p orbitals (moja kwa kila N) zitaelekezwa mwisho hadi mwisho na zitaunda dhamana ya σ.
 " height="174" width="442" src="https://chem.libretexts.org/@api/dek...ewStru_img.jpg">
" height="174" width="442" src="https://chem.libretexts.org/@api/dek...ewStru_img.jpg">
8. (a) 2 σ 2 π;
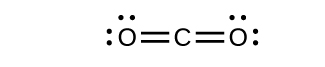
(b) 1 σ 2 π;
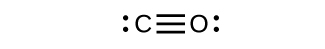
8.2: Orbitals ya Atomiki ya Mseto
Kemia Mwisho wa Sura ya Mazoezi
Kwa nini dhana ya mahuluti inahitajika katika nadharia ya dhamana ya valence?
Hybridization ni kuletwa kueleza jiometri ya obitals bonding katika valance dhamana nadharia.
Kutoa sura inayoelezea kila seti ya orbital ya mseto:
(a) sp 2
(b) sp 3 d
(c) sp
(d) sp 3 d 2
Eleza kwa nini atomu ya kaboni haiwezi kuunda vifungo tano kwa kutumia sp 3 d orbitals mseto.
Hakuna orbitals d katika shell valence ya kaboni.
Je, ni mahuluti ya atomi ya kati katika kila moja ya yafuatayo?
(a) BeH 2
(b) SF 6
(c)\(\ce{PO4^3-}\)
(d) PCL 5
Molekuli yenye formula AB 3 inaweza kuwa na moja ya maumbo manne tofauti. Kutoa sura na hybridization ya kati atomi A kwa kila.
Mpango wa trigonal, sp 2; piramidi ya trigonal (jozi moja pekee kwenye A) sp 3; T-umbo (jozi mbili za pekee kwenye A sp 3 d, au (jozi tatu za pekee kwenye A) sp 3 d 2
Methionine, CH 3 SCH 2 CH 2 CH (NH 2) CO 2 H, ni asidi amino inayopatikana katika protini. Chora muundo wa Lewis wa kiwanja hiki. Aina ya hybridization ya kila kaboni, oksijeni, nitrojeni, na sulfuri ni nini?

Asidi ya sulfuriki hutengenezwa na mfululizo wa athari zinazowakilishwa na equations zifuatazo:
\(\ce{S8}(s)+\ce{8O2}(g)⟶\ce{8SO2}(g)\)
\(\ce{2SO2}(g)+\ce{O2}(g)⟶\ce{2SO3}(g)\)
\(\ce{SO3}(g)+\ce{H2O}(l)⟶\ce{H2SO4}(l)\)
Draw a Lewis structure, predict the molecular geometry by VSEPR, and determine the hybridization of sulfur for the following:
(a) circular S8 molecule
(b) SO2 molecule
(c) SO3 molecule
(d) H2SO4 molecule (the hydrogen atoms are bonded to oxygen atoms)
(a) Each S has a bent (109°) geometry, sp3

(b) Bent (120°), sp 2

(c) Mpango wa Trigonal, sp 2

(d) Tetrahedral, sp 3

Kemikali mbili muhimu za viwanda, ethene, C 2 H 4, na propene, C 3 H 6, zinazalishwa na mchakato wa kupoteza mvuke (au mafuta):
\(\ce{2C3H8}(g)⟶\ce{C2H4}(g)+\ce{C3H6}(g)+\ce{CH4}(g)+\ce{H2}(g)\)For each of the four carbon compounds, do the following:
(a) Draw a Lewis structure.
(b) Predict the geometry about the carbon atom.
(c) Determine the hybridization of each type of carbon atom.
For many years after they were discovered, it was believed that the noble gases could not form compounds. Now we know that belief to be incorrect. A mixture of xenon and fluorine gases, confined in a quartz bulb and placed on a windowsill, is found to slowly produce a white solid. Analysis of the compound indicates that it contains 77.55% Xe and 22.45% F by mass.
(a) What is the formula of the compound?
(b) Write a Lewis structure for the compound.
(c) Predict the shape of the molecules of the compound.
(d) What hybridization is consistent with the shape you predicted?
(a) XeF2
(b)

(c) mstari (d) sp 3 d
Fikiria asidi ya nitrous, HNO 2 (HONO).
(a) Andika muundo Lewis.
(b) Je, ni jozi ya elektroni na jiometri za molekuli za ndani za oksijeni na atomi za nitrojeni katika molekuli ya HNO 2?
(c) ni hybridization juu ya ndani oksijeni na nitrojeni atomi katika HNO 2 nini?
Mgomo-popote mechi zina safu ya KClo 3 na safu ya P 4 S 3. Joto lililozalishwa na msuguano wa kushangaza mechi husababisha misombo hii miwili kuitikia kwa nguvu, ambayo huweka moto kwenye shina la mbao la mechi. Clo 3 ina\(\ce{ClO3-}\) ion. P 4 S 3 ni molekuli isiyo ya kawaida na muundo wa mifupa.

(a) Andika miundo Lewis kwa P 4 S 3 na\(\ce{ClO3-}\) ion.
(b) Describe the geometry about the P atoms, the S atom, and the Cl atom in these species.
(c) Assign a hybridization to the P atoms, the S atom, and the Cl atom in these species.
(d) Determine the oxidation states and formal charge of the atoms in P4S3 and the \(\ce{ClO3-}\) ion.
(a)

(b) P atomi, piramidi ya trigonal; S atomi, bent, na jozi mbili pekee; Atomi za Cl, piramidi ya trigonal; (c) Uchanganyiko kuhusu P, S, na Cl ni, katika hali zote, sp 3; (d) Oxidation inasema P +1\(−1\dfrac{1}{3}\), S, Cl +5, O -2. mashtaka rasmi: P 0; S 0; Cl +2: The -1
Kutambua hybridization ya kila atomi kaboni katika molekuli zifuatazo. (Mpangilio wa atomi hutolewa; unahitaji kuamua ngapi vifungo vinavyounganisha kila jozi ya atomi.)

Andika Lewis miundo kwa NF 3 na PF 5. Kwa misingi ya orbitals ya mseto, kuelezea ukweli kwamba NF 3, PF 3, na PF 5 ni molekuli imara, lakini NF 5 haipo.

Phosphorus na nitrojeni zinaweza kuunda mahuluti ya sp 3 kuunda vifungo vitatu na kushikilia jozi moja pekee katika PF 3 na NF 3, kwa mtiririko huo. Hata hivyo, nitrojeni haina orbitals valence d, hivyo haiwezi kuunda seti ya orbitals sp 3 d mseto kumfunga atomi tano za fluorini katika NF 5. Phosphorus ina orbitals d na inaweza kumfunga atomi tano za fluorini na sp 3 d orbitals mseto katika PF 5.
Mbali na NF 3, derivatives nyingine mbili za fluoro za nitrojeni zinajulikana: N 2 F 4 na N 2 F 2. Ni maumbo gani unayotabiri kwa molekuli hizi mbili? Je, ni hybridization kwa nitrojeni katika kila molekuli?
8.3: Vifungo vingi
Kemia Mwisho wa Sura ya Mazoezi
Nishati ya dhamana ya dhamana ya C—C moja ina wastani wa 347 kJ mol -1; ile ya dhamana ya CC mara tatu wastani wa 839 kJ mol -1. Eleza kwa nini dhamana ya mara tatu si mara tatu kama nguvu kama dhamana moja.
Dhamana ya mara tatu ina dhamana moja σ na vifungo viwili π. Dhamana ya σ ina nguvu kuliko dhamana π kutokana na mwingiliano mkubwa zaidi.
Kwa ion carbonate,\(\ce{CO3^2-}\), draw all of the resonance structures. Identify which orbitals overlap to create each bond.
A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, H3CCN. It is present in paint strippers.
(a) Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule.
(b) Identify the hybrid orbitals used by the carbon atoms in the molecule to form σ bonds.
(c) Describe the atomic orbitals that form the π bonds in the molecule. Note that it is not necessary to hybridize the nitrogen atom.
(a)

(b) atomi ya kaboni ya terminal inatumia orbitals ya mseto wa sp 3, wakati atomi ya kaboni ya kati ni sp iliyochanganywa. (c) Kila moja ya vifungo viwili π hutengenezwa na kuingiliana kwa 2 p orbital juu ya kaboni na nitrojeni 2 p orbital.
Kwa molekuli allene,\(\mathrm{H_2C=C=CH_2}\), kutoa hybridization ya kila atomi kaboni. Je! Atomi za hidrojeni zitakuwa katika ndege moja au ndege za perpendicular?
Tambua mahuluti ya atomi ya kati katika kila moja ya molekuli na ions zifuatazo zilizo na vifungo vingi:
(a) ClNO (N ni atomi ya kati)
(b) CS 2
(c) Cl 2 CO (C ni atomi ya kati)
(d) Cl 2 SO (S ni atomi ya kati)
(e) SO 2 F 2 (S ni atomi ya kati)
(f) Xeo 2 F 2 (Xe ni atomi ya kati)
(g)\(\ce{ClOF2+}\) (Cl ni atomi kuu)
(a) sp 2; (b) sp; (c) sp 2; (d) sp 3; (e) sp 3; (f) sp 3 d; (g) sp 3
Eleza jiometri ya Masi na mahuluti ya atomi za N, P, au S katika kila moja ya misombo ifuatayo.
(a) H 3 PO 4, asidi fosforasi, kutumika katika vinywaji cola laini
(b) NH 4 NO 3, nitrati amonia, mbolea na kulipuka
(c) S 2 Cl 2, dichloride disulfuri, kutumika katika mpira vulcanizing
(d) K 4 [The 3 POPO 3], potassium pyrophosphate, ingredient katika baadhi toothpastes
Kwa kila moja ya molekuli zifuatazo, zinaonyesha uharibifu ulioombwa na ikiwa elektroni zitaondolewa:
(a) ozoni (O 3) kati O hybridization
(b) dioksidi kaboni (CO 2) kati ya C hybridization
(c) nitrojeni dioksidi (NO 2) kati N hybridization
(d) phosphate ion (\(\ce{PO4^3-}\)) kati P hybridization
(a) sp 2, delocalized; (b) sp, localized; (c) sp 2, delocalized; (d) sp 3, delocalized
Kwa kila moja ya miundo ifuatayo, tambua uharibifu ulioombwa na kama elektroni zitafutwa:
(a) Hybridization ya kila kaboni

(b) Hybridization ya sulfuri

(c) Atomi zote

Chora mchoro orbital kwa kaboni katika CO 2 kuonyesha jinsi wengi kaboni atomi elektroni ni katika kila orbital.

Kila moja ya elektroni nne iko katika orbital tofauti na huingiliana na elektroni kwenye atomi ya oksijeni.
8.4: Nadharia ya Orbital ya Masi
Kemia Mwisho wa Sura ya Mazoezi
Mchoro usambazaji wa wiani wa elektroni katika bonding na antibonding orbitals Masi sumu kutoka orbitals mbili s na kutoka orbitals mbili p.
Je! Yafuatayo ni sawa, na yanatofautianaje?
(a) σ orbitals Masi na π orbitals Masi
(b) kwa orbital ya atomiki na kwa orbital ya Masi
(c) obitals bonding na orbitals antibonding
(a) kufanana: Wote ni bonding orbitals ambayo inaweza kuwa na upeo wa elektroni mbili. Tofauti: σ orbitals ni mchanganyiko wa mwisho hadi mwisho wa orbitals atomiki, wakati π orbitals huundwa na kuingiliana upande kwa upande wa orbitals. (b) Kufanana: Wote ni miundo ya quantum-mitambo ambayo inawakilisha uwezekano wa kutafuta elektroni kuhusu atomi au molekuli. Tofauti: kwa orbital atomia inaeleza tabia ya elektroni moja tu kwa wakati kulingana na atomu. Kwa molekuli, inawakilisha mchanganyiko wa hisabati wa orbitals atomiki. (c) kufanana: Wote ni orbitals ambayo inaweza kuwa na elektroni mbili. Tofauti: obiti za kuunganisha husababisha kushikilia atomi mbili au zaidi pamoja. Orbitals ya antibonding ina athari ya kuharibu uhusiano wowote uliofanyika.
Ikiwa orbitals Masi huundwa kwa kuchanganya orbitals atomiki tano kutoka atomi A na orbitals atomiki tano kutoka atomi B kuchanganya, ni wangapi orbitals Masi kusababisha?
Je, molekuli yenye idadi isiyo ya kawaida ya elektroni inaweza kuwa diamagnetic? Eleza kwa nini au kwa nini.
Idadi isiyo ya kawaida ya elektroni haiwezi kuunganishwa, bila kujali utaratibu wa orbitals ya Masi. Itakuwa daima kuwa paramagnetic.
Je, molekuli yenye idadi ya elektroni inaweza kuwa paramagnetic? Eleza kwa nini au kwa nini.
Kwa nini bonding orbitals Masi chini katika nishati kuliko orbitals mzazi atomiki?
Orbitals ya kuunganisha ina wiani wa elektroni karibu na kiini zaidi ya moja. Uingiliano kati ya nuclei ya kushtakiwa vyema na elektroni za kushtakiwa vibaya huimarisha mfumo.
Tumia utaratibu wa dhamana kwa ion na usanidi huu:
Explain why an electron in the bonding molecular orbital in the H2 molecule has a lower energy than an electron in the 1s atomic orbital of either of the separated hydrogen atoms.
The pairing of the two bonding electrons lowers the energy of the system relative to the energy of the nonbonded electrons.
Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions.
(a) \(\ce{Na2^2+}\)
(b) \(\ce{Mg2^2+}\)
(c) \(\ce{Al2^2+}\)
(d) \(\ce{Si2^2+}\)
(e) \(\ce{P2^2+}\)
(f) \(\ce{S2^2+}\)
(g) \(\ce{F2^2+}\)
(h) \(\ce{Ar2^2+}\)
Determine the bond order of each member of the following groups, and determine which member of each group is predicted by the molecular orbital model to have the strongest bond.
(a) H2, \(\ce{H2+}\), \(\ce{H2-}\)
(b) O2, \(\ce{O2^2+}\), \(\ce{O2^2-}\)
(c) Li2, \(\ce{Be2+}\), Be2
(d) F2, \(\ce{F2+}\), \(\ce{F2-}\)
(e) N2, \(\ce{N2+}\), \(\ce{N2-}\)
(a) H2 bond order = 1, \(\ce{H2+}\) bond order = 0.5, \(\ce{H2-}\) bond order = 0.5, strongest bond is H2; (b) O2 bond order = 2, \(\ce{O2^2+}\) bond order = 3; \(\ce{O2^2-}\) bond order = 1, strongest bond is \(\ce{O2^2+}\); (c) Li2 bond order = 1, \(\ce{Be2+}\) bond order = 0.5, Be2 bond order = 0, strongest bond is \(\ce{Li2}\);(d) F2 bond order = 1, \(\ce{F2+}\) bond order = 1.5, \(\ce{F2-}\) bond order = 0.5, strongest bond is \(\ce{F2+}\); (e) N2 bond order = 3, \(\ce{N2+}\) bond order = 2.5, \(\ce{N2-}\) bond order = 2.5, strongest bond is N2
For the first ionization energy for an N2 molecule, what molecular orbital is the electron removed from?
Compare the atomic and molecular orbital diagrams to identify the member of each of the following pairs that has the highest first ionization energy (the most tightly bound electron) in the gas phase:
(a) H and H2
(b) N and N2
(c) O and O2
(d) C and C2
(e) B and B2
(a) H2; (b) N2; (c) O; (d) C2; (e) B2
Which of the period 2 homonuclear diatomic molecules are predicted to be paramagnetic?
A friend tells you that the 2s orbital for fluorine starts off at a much lower energy than the 2s orbital for lithium, so the resulting σ2s molecular orbital in F2 is more stable than in Li2. Do you agree?
Yes, fluorine is a smaller atom than Li, so atoms in the 2s orbital are closer to the nucleus and more stable.
True or false: Boron contains 2s22p1 valence electrons, so only one p orbital is needed to form molecular orbitals.
What charge would be needed on F2 to generate an ion with a bond order of 2?
2+
Predict whether the MO diagram for S2 would show s-p mixing or not.
Explain why \(\ce{N2^2+}\) is diamagnetic, while \(\ce{O2^4+}\), which has the same number of valence electrons, is paramagnetic.
N2 has s-p mixing, so the π orbitals are the last filled in \(\ce{N2^2+}\). O2 does not have s-p mixing, so the σp orbital fills before the π orbitals.
Using the MO diagrams, predict the bond order for the stronger bond in each pair:
(a) B2 or \(\ce{B2+}\)
(b) F2 or \(\ce{F2+}\)
(c) O2 or \(\ce{O2^2+}\)
(d) \(\ce{C2+}\) or \(\ce{C2-}\)


