16E: Thermodynamics (Mazoezi)
- Page ID
- 175776
16.1: Mazoezi ya Spontaneity
Q16.1.1
Je, ni mmenyuko wa pekee?
S16.1.1
Mmenyuko una tabia ya asili ya kutokea na hufanyika bila pembejeo ya nishati ya kuendelea kutoka chanzo cha nje.
Q16.1.2
Je, ni mmenyuko usio na kawaida?
Q16.1.3
Eleza kama michakato ifuatayo ni ya pekee au isiyo ya kawaida.
- Maji ya maji ya kufungia kwenye joto chini ya kiwango chake cha kufungia
- Maji ya maji ya kufungia kwenye joto la juu ya kiwango chake cha kufungia
- Mwako wa petroli
- Mpira uliotupwa hewa
- Raindrop kuanguka chini
- Iron kutu katika hali ya unyevu
S16.1.2
kwa hiari; bila ya kawaida; kwa hiari; bila ya kawaida; kwa hiari; kwa hiari
Q16.1.4
Helium kujazwa puto kuwaka deflates mara moja kama Yeye atomi kueneza kwa njia ya ukuta wa puto. Eleza ugawaji wa suala na/au nishati inayoambatana na mchakato huu.
Q16.1.5
Vifaa vingi vya plastiki ni polima za kikaboni ambazo zina kaboni na hidrojeni. Oxidation ya plastiki hizi katika hewa kuunda dioksidi kaboni na maji ni mchakato wa hiari; hata hivyo, vifaa vya plastiki huwa na kuendelea katika mazingira. Eleza.
S16.1.5
Ingawa oxidation ya plastiki ni ya pekee, kiwango cha oxidation ni polepole sana. Kwa hiyo plastiki ni kinetically imara na wala kuoza appreciably hata kwa muda mrefu kiasi cha muda.
16.2: Mazoezi ya Entropy
Q16.2.1
Katika chini Kielelezo mgawanyo wote iwezekanavyo na microstates ni umeonyesha kwa chembe nne tofauti pamoja kati ya masanduku mawili. Kuamua mabadiliko entropy, Δ S, kama chembe awali ni sawasawa kusambazwa kati ya masanduku mawili, lakini juu ya ugawaji wote kuishia katika Box (b).
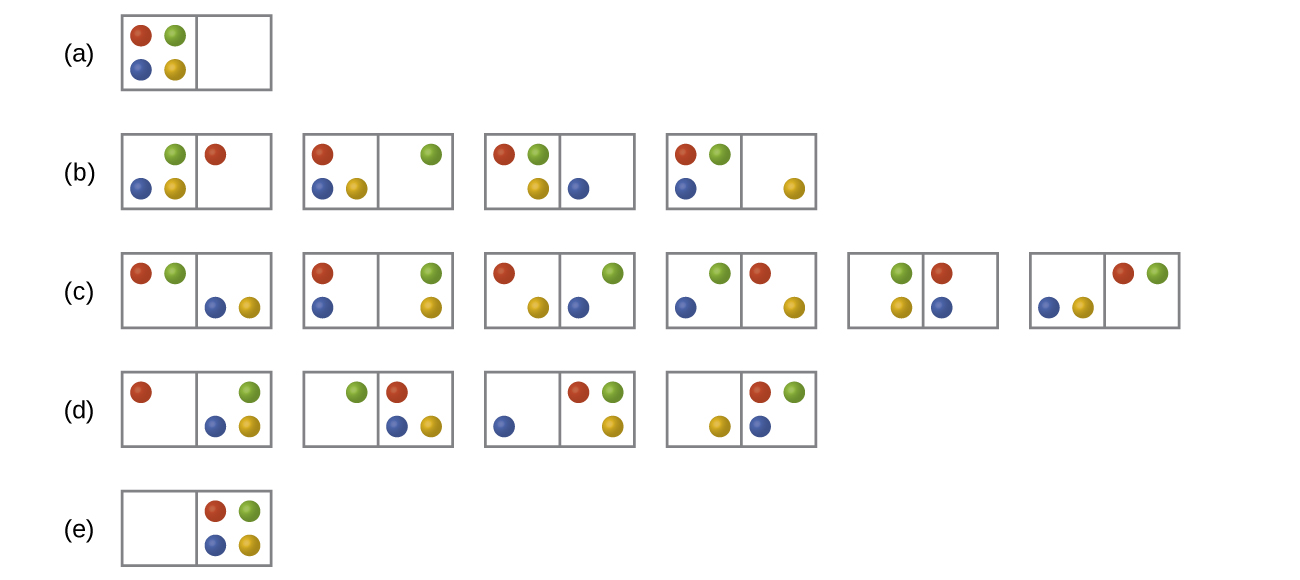
Q16.2.2
Katika Kielelezo wote wa mgawanyo iwezekanavyo na microstates ni umeonyesha kwa chembe nne tofauti pamoja kati ya masanduku mawili. Kuamua mabadiliko ya entropy, Δ S, kwa mfumo wakati inabadilishwa kutoka usambazaji hadi usambazaji (d).

S16.2.2
Kuna microstates nne za awali na microstates nne za mwisho.
\[ΔS=k\ln\dfrac{W_\ce{f}}{W_\ce{i}}=\mathrm{1.38×10^{−23}\:J/K×\ln\dfrac{4}{4}=0}\]
Q16.2.3
Je, mchakato ulioelezwa katika kipengee kilichopita unahusianaje na mfumo ulioonyeshwa kwenye [kiungo]?
Q16.2.4
Fikiria mfumo unaofanana na ule ulio chini, isipokuwa kuwa una chembe sita badala ya nne. Je! Ni uwezekano gani wa kuwa na chembe zote katika moja tu ya masanduku mawili katika kesi hiyo? Linganisha hili na uwezekano sawa kwa mfumo wa chembe nne ambazo tumepata kuwa sawa na\(\dfrac{1}{8}\). Ulinganisho huu unatuambia nini kuhusu mifumo kubwa zaidi?

S16.2.4
Uwezekano wa chembe zote kuwa upande mmoja ni\(\dfrac{1}{32}\). Uwezekano huu unaonekana chini kuliko\(\dfrac{1}{8}\) matokeo ya mfumo wa chembe nne. Hitimisho tunaweza kufanya ni kwamba uwezekano wa chembe zote kukaa katika sehemu moja tu ya mfumo utapungua kwa kasi kadiri idadi ya chembe inavyoongezeka, na, kwa mfano, uwezekano wa molekuli zote za gesi kukusanya katika upande mmoja tu wa chumba kwenye joto la kawaida na shinikizo ni kidogo tangu idadi ya molekuli gesi katika chumba ni kubwa sana.
Q16.2.5
Fikiria mfumo umeonyeshwa kwenye Kielelezo. Je! Ni mabadiliko gani katika entropy kwa mchakato ambapo nishati inahusishwa tu na chembe A, lakini katika hali ya mwisho nishati inasambazwa kati ya chembe mbili tofauti?

Q16.2.6
Fikiria mfumo umeonyeshwa kwenye Kielelezo. Je! Ni mabadiliko gani katika entropy kwa mchakato ambapo nishati inahusishwa na chembe A na B, na nishati inasambazwa kati ya chembe mbili katika masanduku tofauti (moja katika A-B, nyingine katika C-D)?
S16.2.6
Kuna hali moja tu ya awali. Kwa hali ya mwisho, nishati inaweza kuwa katika jozi A-C, A-D, B-C, au B-D. Hivyo, kuna majimbo manne ya mwisho iwezekanavyo.
\[ΔS=k\ln\left(\dfrac{W_\ce{f}}{W_\ce{i}}\right)=\mathrm{1.38×10^{−23}\:J/K×\ln\left(\dfrac{4}{1}\right)=1.91×10^{−23}\:J/K}\]
Q16.2.7
Panga seti zifuatazo za mifumo ili ya kuongezeka kwa entropy. Fikiria mole moja ya kila dutu na joto sawa kwa kila mwanachama wa seti.
- H (2 g), Bro (4g), HbR (g)
- H 2 O (l), H 2 O (g), H 2 O (s)
- Yeye (g), Cl 2 (g), P 4 (g)
Q16.2.8
Katika joto la kawaida, entropy ya halojeni huongezeka kutoka I 2 hadi Br 2 hadi Cl 2. Eleza.
S16.2.8
Misa ya molekuli hizi ingeonyesha mwenendo kinyume katika entropies yao. Mwelekeo uliozingatiwa ni matokeo ya tofauti kubwa zaidi ya entropy na hali ya kimwili. Katika joto la kawaida, I 2 ni imara, Br 2 ni kioevu, na Cl 2 ni gesi.
Q16.2.9
Fikiria michakato miwili: upungufu wa I 2 (s) na kiwango cha I 2 (s) (Kumbuka: mchakato wa mwisho unaweza kutokea kwa joto sawa lakini shinikizo la juu).
\[\ce{I2}(s)⟶\ce{I2}(g)\]
\[\ce{I2}(s)⟶\ce{I2}(l)\]
Je, Δ S ni chanya au hasi katika michakato hii? Ni ipi kati ya taratibu ambazo ukubwa wa mabadiliko ya entropy utakuwa mkubwa zaidi?
Q16.2.11
Eleza dutu gani katika jozi zilizopewa ina thamani ya juu ya entropy. Eleza uchaguzi wako.
- C 2 H 5 OH (l) au C 3 H 7 OH (l)
- C 2 H 5 OH (l) au C 2 H 5 OH (g)
- Saa 2H (g)
S16.2.11
C 3 H 7 OH (l) kama ni molekuli kubwa (ngumu zaidi na kubwa zaidi), na hivyo microstates zaidi kuelezea mwendo wake zinapatikana kwa joto lolote. C 2 H 5 OH (g) kama ilivyo katika hali ya gesi. 2H (g), tangu entropy ni mali kubwa, na hivyo atomi mbili za H (au moles mbili za atomi H) zina entropy mara mbili kama atomi moja (au mole moja ya atomi).
Q16.2.11
Kutabiri ishara ya mabadiliko ya entropy kwa michakato ifuatayo:
- Mchemraba wa barafu hupunguzwa karibu na kiwango chake cha kuyeyuka.
- Pumzi ya kupumua huunda ukungu asubuhi ya baridi.
- Theluji huyeyuka.
Q16.2.12
Kutabiri ishara ya mabadiliko ya enthalpy kwa michakato ifuatayo. Kutoa sababu ya utabiri wako.
- \(\ce{Pb^2+}(aq)+\ce{S^2-}(aq)⟶\ce{PbS}(s)\)
- \(\ce{2Fe}(s)+\ce{3O2}(g)⟶\ce{Fe2O3}(s)\)
- \(\ce{2C6H14}(l)+\ce{19O2}(g)⟶\ce{14H2O}(g)+\ce{12CO2}(g)\)
S16.2.12
Hasi. Kuzuia imara kwa kiasi kikubwa kunapungua idadi ya ions za simu katika suluhisho. Hasi. Kuna hasara halisi ya moles tatu za gesi kutoka kwa reactants kwa bidhaa. Chanya. Kuna ongezeko la wavu la moles saba za gesi kutoka kwa reactants kwa bidhaa.
Q16.2.13
Andika usawa wa kemikali kwa mwako wa methane, CH 4 (g), kutoa dioksidi kaboni na mvuke wa maji. Eleza kwa nini ni vigumu kutabiri kama Δ S ni chanya au hasi kwa mmenyuko huu wa kemikali.
Q16.2.14
Andika usawa wa kemikali kwa mwako wa benzini, C 6 H 6 (l), kutoa dioksidi kaboni na mvuke wa maji. Je, unatarajia Δ S kuwa chanya au hasi katika mchakato huu?
S16.2.14
\[\ce{C6H6}(l)+7.5\ce{O2}(g)⟶\ce{3H2O}(g)+\ce{6CO2}(g)\]
Kuna moles 7.5 ya gesi awali, na 3 + 6 = 9 moles ya gesi mwishoni. Kwa hiyo, inawezekana kwamba entropy huongezeka kama matokeo ya mmenyuko huu, na Δ S ni chanya.
16.3: Sheria ya Pili na ya Tatu
Q16.3.0
Ni tofauti gani kati ya Δ S, Δ S°, na\(ΔS^\circ_{298}\) kwa mabadiliko ya kemikali?
Q16.3.1
Tumia\(ΔS^\circ_{298}\) mabadiliko yafuatayo.
- \(\ce{SnCl4}(l)⟶\ce{SnCl4}(g)\)
- \(\ce{CS2}(g)⟶\ce{CS2}(l)\)
- \(\ce{Cu}(s)⟶\ce{Cu}(g)\)
- \(\ce{H2O}(l)⟶\ce{H2O}(g)\)
- \(\ce{2H2}(g)+\ce{O2}(g)⟶\ce{2H2O}(l)\)
- \(\ce{2HCl}(g)+\ce{Pb}(s)⟶\ce{PbCl2}(s)+\ce{H2}(g)\)
- \(\ce{Zn}(s)+\ce{CuSO4}(s)⟶\ce{Cu}(s)+\ce{ZnSO4}(s)\)
S16.3.1
107 J/K; -86.4 J/K; 133.2 J/K; 118.8 J/K; -326.6 J/K; -171.9 J/K; (g) -7.2 J/K
Q16.3.2
Kuamua mabadiliko ya entropy kwa mwako wa ethanol ya kioevu, C 2 H 5 OH, chini ya hali ya hali ya kawaida kutoa dioksidi kaboni ya gesi na maji ya maji.
Q16.3.3
Kuamua mabadiliko ya entropy kwa mwako wa propane ya gesi, C 3 H 8, chini ya hali ya kawaida ya kutoa gesi dioksidi kaboni na maji.
S16.3.3
100.6 J/K
Q16.3.4
Athari za “Thermite” zimetumika kwa kulehemu sehemu za chuma kama vile reli za reli na katika kusafisha chuma. Moja ya mmenyuko huo wa thermite ni:
\[\ce{Fe2O3}(s)+\ce{2Al}(s)⟶\ce{Al2O3}(s)+\ce{2Fe}(s)\]
Je, mmenyuko hupatikana kwa joto la kawaida chini ya hali ya kawaida? Wakati wa majibu, mazingira hupata 851.8 kJ/mol ya joto.
Q16.3.5
Kutumia\(S^\circ_{298}\) maadili husika yaliyoorodheshwa katika Kiambatisho G, hesabu\(S^\circ_{298}\) kwa mabadiliko yafuatayo:
- \(\ce{N2}(g)+\ce{3H2}(g)⟶\ce{2NH3}(g)\)
- \(\ce{N2}(g)+\dfrac{5}{2}\ce{O2}(g)⟶\ce{N2O5}(g)\)
S16.3.5
-198.1 J/K; -348.9 J/K
Q16.3.6
Kutoka kwa habari zifuatazo, tambua\(ΔS^\circ_{298}\) kwa yafuatayo:
- \(\ce{N}(g)+\ce{O}(g)⟶\ce{NO}(g) \hspace{20px} ΔS^\circ_{298}=\,?\)
- \(\ce{N2}(g)+\ce{O2}(g)⟶\ce{2NO}(g) \hspace{20px} ΔS^\circ_{298}=\mathrm{24.8\: J/K}\)
- \(\ce{N2}(g)⟶\ce{2N}(g) \hspace{20px} ΔS^\circ_{298}=\mathrm{115.0\: J/K}\)
- \(\ce{O2}(g)⟶\ce{2O}(g) \hspace{20px} ΔS^\circ_{298}=\mathrm{117.0\: J/K}\)
Q16.3.7
Kwa kuhesabu Δ S univ katika kila joto, onyesha kama kuyeyuka kwa mole 1 ya NaCl (s) ni hiari saa 500 °C na saa 700 °C.
\[S^\circ_{\ce{NaCl}(s)}=\mathrm{72.11\:\dfrac{J}{mol⋅K}}\hspace{40px} S^\circ_{\ce{NaCl}(l)}=\mathrm{95.06\:\dfrac{J}{mol⋅K}}\hspace{40px ΔH^\circ_\ce{fusion}=\mathrm{27.95\: kJ/mol}\]
Ni mawazo gani yanayofanywa kuhusu habari ya thermodynamic (maadili ya entropy na enthalpy) yaliyotumiwa kutatua tatizo hili?
S16.3.7
Kama Δ S univ <0 katika kila moja ya joto hizi, kuyeyuka sio kwa hiari kwa yeyote kati yao. Maadili yaliyotolewa kwa entropy na enthalpy ni kwa NaCl saa 298 K. inadhaniwa kuwa haya hayabadilika kwa kiasi kikubwa katika joto la juu linalotumika katika tatizo.
Q16.3.8
Tumia data ya kawaida ya entropy katika Kiambatisho G ili kuamua mabadiliko katika entropy kwa kila moja ya athari zilizoorodheshwa katika [kiungo]. Zote zinaendeshwa chini ya hali ya kawaida na 25 °C.
Q16.3.8
2.86 J/K; 24.8 J/K; -113.2 J/K; -24.7 J/K; 15.5 J/K; 290.0 J/K
16.4: Nishati ya bure
Q16.4.1
Ni tofauti gani kati ya Δ G, Δ G°, na\(ΔG^\circ_{298}\) kwa mabadiliko ya kemikali?
Q16.4.2
athari ina\(ΔH^\circ_{298}\) = 100 kJ/mol na\(ΔS^\circ_{298}=\textrm{250 J/mol⋅K}\). Je, mmenyuko hupatikana kwa joto la kawaida? Ikiwa sio, chini ya hali gani ya joto itakuwa ya pekee?
S16.4.2
Majibu hayatoshi kwa joto la kawaida. Zaidi ya 400 K, Δ G itakuwa hasi, na majibu yatakuwa ya pekee.
Q16.4.3
Eleza kinachotokea kama mmenyuko huanza na Δ G <0 (hasi) na kufikia hatua ambapo Δ G = 0.
Tumia data ya kawaida ya nishati ya malezi katika Kiambatisho G ili kuamua mabadiliko ya nishati ya bure kwa kila moja ya athari zifuatazo, ambazo zinaendeshwa chini ya hali ya hali ya kawaida na 25 °C Tambua kila mmoja kama ama hiari au isiyo ya kawaida katika hali hizi.
- \(\ce{MnO2}(s)⟶\ce{Mn}(s)+\ce{O2}(g)\)
- \(\ce{H2}(g)+\ce{Br2}(l)⟶\ce{2HBr}(g)\)
- \(\ce{Cu}(s)+\ce{S}(g)⟶\ce{CuS}(s)\)
- \(\ce{2LiOH}(s)+\ce{CO2}(g)⟶\ce{Li2CO3}(s)+\ce{H2O}(g)\)
- \(\ce{CH4}(g)+\ce{O2}(g)⟶\ce{C}(s,\,\ce{graphite})+\ce{2H2O}(g)\)
- \(\ce{CS2}(g)+\ce{3Cl2}(g)⟶\ce{CCl4}(g)+\ce{S2Cl2}(g)\)
S16.4.3
465.1 kJ bila ya kawaida; -106.86 kJ kwa hiari; -53.6 kJ kwa hiari; -83.4 kJ kwa hiari; -406.7 kJ kwa hiari; -30.0 kJ kwa hiari
Q16.4.4
Tumia data ya nishati ya bure ya kiwango katika Kiambatisho G ili kuamua mabadiliko ya nishati ya bure kwa kila moja ya athari zifuatazo, ambazo zinaendeshwa chini ya hali ya kawaida na 25 °C.
- \(\ce{C}(s,\, \ce{graphite})+\ce{O2}(g)⟶\ce{CO2}(g)\)
- \(\ce{O2}(g)+\ce{N2}(g)⟶\ce{2NO}(g)\)
- \(\ce{2Cu}(s)+\ce{S}(g)⟶\ce{Cu2S}(s)\)
- \(\ce{CaO}(s)+\ce{H2O}(l)⟶\ce{Ca(OH)2}(s)\)
- \(\ce{Fe2O3}(s)+\ce{3CO}(g)⟶\ce{2Fe}(s)+\ce{3CO2}(g)\)
- \(\ce{CaSO4⋅2H2O}(s)⟶\ce{CaSO4}(s)+\ce{2H2O}(g)\)
Kutokana na:
\[\ce{P4}(s)+\ce{5O2}(g)⟶\ce{P4O10}(s) \hspace{20px} ΔG^\circ_{298}=\mathrm{−2697.0\: kJ/mol}\]
\[\ce{2H2}(g)+\ce{O2}(g)⟶\ce{2H2O}(g) \hspace{20px} ΔG^\circ_{298}=\mathrm{−457.18\: kJ/mol}\]
\[\ce{6H2O}(g)+\ce{P4O10}(g)⟶\ce{4H3PO4}(l) \hspace{20px} ΔG^\circ_{298}=\mathrm{−428.66\: kJ/mol}\]
Q16.4.5
- Kuamua nishati ya kawaida ya malezi\(ΔG^\circ_\ce{f}\), kwa asidi ya fosforasi.
- Matokeo yako ya mahesabu yanalinganishaje na thamani katika Kiambatisho G? Eleza.
S16.4.5
-1124.3 KJ/mol kwa mabadiliko ya kawaida ya nishati ya bure. Hesabu inakubaliana na thamani katika Kiambatisho G kwa sababu nishati ya bure ni kazi ya serikali (kama vile enthalpy na entropy), hivyo mabadiliko yake yanategemea tu majimbo ya awali na ya mwisho, si njia kati yao.
Q16.4.6
Je! Kuundwa kwa ozoni (O 3 (g)) kutoka kwa oksijeni (O 2 (g)) kwa hiari kwenye joto la kawaida chini ya hali ya hali ya kawaida?
Q16.4.7
Fikiria uharibifu wa zebaki nyekundu (II) oksidi chini ya hali ya hali ya kawaida.
\[\ce{2HgO}(s,\,\ce{red})⟶\ce{2Hg}(l)+\ce{O2}(g)\]
- Je, kuharibika kwa hiari chini ya hali ya hali ya kawaida?
- Juu ya joto gani majibu huwa ya pekee?
S16.4.7
Mitikio ni yasiyo ya kawaida; Zaidi ya 566 °C mchakato ni wa hiari.
Q16.4.8
Miongoni mwa mambo mengine, mafuta bora kwa ajili ya kudhibiti trusters ya gari nafasi lazima kuoza katika mmenyuko hiari exothermic wakati wazi kwa kichocheo sahihi. Kutathmini dutu zifuatazo chini ya hali ya kiwango hali kama wagombea kufaa kwa ajili ya nishati.
- Amonia:\(\ce{2NH3}(g)⟶\ce{N2}(g)+\ce{3H2}(g)\)
- Diborane:\(\ce{B2H6}(g)⟶\ce{2B}(g)+\ce{3H2}(g)\)
- Hydrazine:\(\ce{N2H4}(g)⟶\ce{N2}(g)+\ce{2H2}(g)\)
- Peroxide ya hidrojeni\(\ce{H2O2}(l)⟶\ce{H2O}(g)+\dfrac{1}{2}\ce{O2}(g)\)
Q16.4.9
Tumia Δ G° kwa kila moja ya athari zifuatazo kutoka mara kwa mara ya usawa kwenye joto lililopewa.
- \(\ce{N2}(g)+\ce{O2}(g)⟶\ce{2NO}(g) \hspace{20px} \mathrm{T=2000\:°C} \hspace{20px} K_p=4.1×10^{−4}\)
- \(\ce{H2}(g)+\ce{I2}(g)⟶\ce{2HI}(g) \hspace{20px} \mathrm{T=400\:°C} \hspace{20px} K_p=50.0\)
- \(\ce{CO2}(g)+\ce{H2}(g)⟶\ce{CO}(g)+\ce{H2O}(g) \hspace{20px} \mathrm{T=980\:°C} \hspace{20px} K_p=1.67\)
- \(\ce{CaCO3}(s)⟶\ce{CaO}(s)+\ce{CO2}(g) \hspace{20px} \mathrm{T=900\:°C} \hspace{20px} K_p=1.04\)
- \(\ce{HF}(aq)+\ce{H2O}(l)⟶\ce{H3O+}(aq)+\ce{F-}(aq) \hspace{20px} \mathrm{T=25\:°C} \hspace{20px} K_p=7.2×10^{−4}\)
- \(\ce{AgBr}(s)⟶\ce{Ag+}(aq)+\ce{Br-}(aq) \hspace{20px} \mathrm{T=25\:°C} \hspace{20px} K_p=3.3×10^{−13}\)
S16.4.9
1.5 × 10 2 KJ; -21.9 KJ; -5.34 KJ; -0.383 KJ; 18 kJ; 71 kJ
Q16.4.10
Tumia Δ G° kwa kila moja ya athari zifuatazo kutoka mara kwa mara ya usawa kwenye joto lililopewa.
- \(\ce{Cl2}(g)+\ce{Br2}(g)⟶\ce{2BrCl}(g) \hspace{20px} \mathrm{T=25\:°C} \hspace{20px} K_p=4.7×10^{−2}\)
- \(\ce{2SO2}(g)+\ce{O2}(g)⇌\ce{2SO3}(g) \hspace{20px} \mathrm{T=500\:°C} \hspace{20px} K_p=48.2\)
- \(\ce{H2O}(l)⇌\ce{H2O}(g) \hspace{20px} \mathrm{T=60\:°C} \hspace{20px} K_p=\mathrm{0.196\: atm}\)
- \(\ce{CoO}(s)+\ce{CO}(g)⇌\ce{Co}(s)+\ce{CO2}(g) \hspace{20px} \mathrm{T=550\:°C} \hspace{20px} K_p=4.90×10^2\)
- \(\ce{CH3NH2}(aq)+\ce{H2O}(l)⟶\ce{CH3NH3+}(aq)+\ce{OH-}(aq) \hspace{20px} \mathrm{T=25\:°C} \hspace{20px} K_p=4.4×10^{−4}\)
- \(\ce{PbI2}(s)⟶\ce{Pb^2+}(aq)+\ce{2I-}(aq) \hspace{20px} \mathrm{T=25\:°C} \hspace{20px} K_p=8.7×10^{−9}\)
Q16.4.11
Tumia mara kwa mara ya usawa saa 25 °C kwa kila moja ya athari zifuatazo kutokana na thamani ya Δ G° iliyotolewa.
- \(\ce{O2}(g)+\ce{2F2}(g)⟶\ce{2OF2}(g) \hspace{20px} ΔG°=\mathrm{−9.2\: kJ}\)
- \(\ce{I2}(s)+\ce{Br2}(l)⟶\ce{2IBr}(g) \hspace{20px} ΔG°=\mathrm{7.3\: kJ}\)
- \(\ce{2LiOH}(s)+\ce{CO2}(g)⟶\ce{Li2CO3}(s)+\ce{H2O}(g) \hspace{20px} ΔG°=\mathrm{−79\: kJ}\)
- \(\ce{N2O3}(g)⟶\ce{NO}(g)+\ce{NO2}(g) \hspace{20px} ΔG°=\mathrm{−1.6\: kJ}\)
- \(\ce{SnCl4}(l)⟶\ce{SnCl4}(l) \hspace{20px} ΔG°=\mathrm{8.0\: kJ}\)
S16.4.11
K = 41; K = 0.053; K = 6.9 × 10 13; K = 1.9; K = 0.04
Q16.4.2
Tumia mara kwa mara ya usawa saa 25 °C kwa kila moja ya athari zifuatazo kutokana na thamani ya Δ G° iliyotolewa.
- \(\ce{I2}(s)+\ce{Cl2}(g)⟶\ce{2ICl}(g) \hspace{20px} ΔG°=\mathrm{−10.88\: kJ}\)
- \(\ce{H2}(g)+\ce{I2}(s)⟶\ce{2HI}(g) \hspace{20px} ΔG°=\mathrm{3.4\: kJ}\)
- \(\ce{CS2}(g)+\ce{3Cl2}(g)⟶\ce{CCl4}(g)+\ce{S2Cl2}(g) \hspace{20px} ΔG°=\mathrm{−39\: kJ}\)
- \(\ce{2SO2}(g)+\ce{O2}(g)⟶\ce{2SO3}(g) \hspace{20px} ΔG°=\mathrm{−141.82\: kJ}\)
- \(\ce{CS2}(g)⟶\ce{CS2}(l) \hspace{20px} ΔG°=\mathrm{−1.88\: kJ}\)
Q16.4.13
Tumia mara kwa mara ya usawa kwenye joto lililopewa.
- (a)\(\ce{O2}(g)+\ce{2F2}(g)⟶\ce{2F2O}(g) \hspace{20px} \mathrm{(T=100\:°C)}\)
- \(\ce{I2}(s)+\ce{Br2}(l)⟶\ce{2IBr}(g) \hspace{20px} \mathrm{(T=0.0\:°C)}\)
- \(\ce{2LiOH}(s)+\ce{CO2}(g)⟶\ce{Li2CO3}(s)+\ce{H2O}(g) \hspace{20px} \mathrm{(T=575\:°C)}\)
- \(\ce{N2O3}(g)⟶\ce{NO}(g)+\ce{NO2}(g) \hspace{20px} \mathrm{(T=−10.0\:°C)}\)
- \(\ce{SnCl4}(l)⟶\ce{SnCl4}(g) \hspace{20px} \mathrm{(T=200\:°C)}\)
S16.4.13
Katika kila moja ya yafuatayo, thamani ya Δ G haipatikani kwa joto la mmenyuko. Kwa hiyo, tunapaswa kuhesabu Δ G° kutoka kwa maadili Δ H° na Δ S° na kisha tuhesabu Δ G kutoka kwa uhusiano ΔG° = ΔH° - TΔS°.
- K = 1.29
- K = 2.51 × 10 ˚ 3
- K = 4.83 × 10 3
- K = 0.219
- K = 16.1
Q16.4.14
Tumia mara kwa mara ya usawa kwenye joto lililopewa.
- (a)\(\ce{I2}(s)+\ce{Cl2}(g)⟶\ce{2ICl}(g) \hspace{20px} \mathrm{(T=100\:°C)}\)
- \(\ce{H2}(g)+\ce{I2}(s)⟶\ce{2HI}(g) \hspace{20px} \mathrm{(T=0.0\:°C)}\)
- \(\ce{CS2}(g)+\ce{3Cl2}(g)⟶\ce{CCl4}(g)+\ce{S2Cl2}(g) \hspace{20px} \mathrm{(T=125\:°C)}\)
- \(\ce{2SO2}(g)+\ce{O2}(g)⟶\ce{2SO3}(g) \hspace{20px} \mathrm{(T=675\:°C)}\)
- \(\ce{CS2}(g)⟶\ce{CS2}(l) \hspace{20px} \mathrm{(T=90\:°C)}\)
Q16.4.15
Fikiria majibu yafuatayo saa 298 K:
\[\ce{N2O4}(g)⇌\ce{2NO2}(g) \hspace{20px} K_P=0.142\]
Je! Ni mabadiliko gani ya nishati ya bure katika joto hili? Eleza kinachotokea kwa mfumo wa awali, ambapo majibu na bidhaa ziko katika majimbo ya kawaida, kama inakaribia usawa.
S16.4.16
Mabadiliko ya nishati ya bure ya kawaida ni\(ΔG^\circ_{298}=−RT\ln K=\mathrm{4.84\: kJ/mol}\). Wakati reactants na bidhaa ziko katika majimbo yao ya kawaida (1 bar au 1 atm), Q = 1. Kama mmenyuko unaendelea kuelekea usawa, mabadiliko ya majibu yanaachwa (kiasi cha bidhaa hupungua wakati kiasi cha reactants kinaongezeka): Q <1, na\(ΔG_{298}\) inakuwa chini ya chanya kama inakaribia sifuri. Katika usawa, Q = K, na Δ G = 0.
Q16.4.17
Kuamua kiwango cha kawaida cha kuchemsha (katika kelvin) ya dichloroethane, CH 2 Cl 2. Pata kiwango halisi cha kuchemsha kwa kutumia mtandao au chanzo kingine, na uhesabu kosa la asilimia katika joto. Eleza tofauti, ikiwa ipo, kati ya maadili mawili.
Q16.4.18
Chini ya hali gani ni ya\(\ce{N2O3}(g)⟶\ce{NO}(g)+\ce{NO2}(g)\) pekee?
S16.4.18
Majibu yatakuwa ya kawaida kwa joto kubwa zaidi ya 287 K.
Q16.4.19
Kwa joto la kawaida, mara kwa mara ya usawa (K w) kwa ajili ya kujitegemea ionization ya maji ni 1.00 × 10 -14. Kutumia habari hii, uhesabu mabadiliko ya nishati ya bure ya kawaida kwa mmenyuko wa maji ya ioni ya hidrojeni na ioni ya hidroksidi ili kuzalisha maji. (Kidokezo: Majibu ni reverse ya mmenyuko wa kujitegemea ionization.)
Q16.4.20
Sulfidi hidrojeni ni uchafuzi unaopatikana katika gesi asilia. Kufuatia kuondolewa kwake, inabadilishwa kuwa sulfuri kwa majibu\(\ce{2H2S}(g)+\ce{SO2}(g)⇌\dfrac{3}{8}\ce{S8}(s,\,\ce{rhombic})+\ce{2H2O}(l)\). Je, ni mara kwa mara ya usawa kwa mmenyuko huu? Je, mmenyuko wa endothermic au exothermic?
S16.4.20
K = 5.35 × 10 15
Mchakato huo ni exothermic.
Q16.4.21
Fikiria uharibifu wa CaCO 3 (s) katika CaO (s) na CO 2 (g). Je, ni shinikizo la sehemu ya usawa wa CO 2 kwenye joto la kawaida?
Q16.4.22
Katika maabara, kloridi hidrojeni (HCl (g)) na amonia (NH 3 (g)) mara nyingi kutoroka kutoka chupa ya ufumbuzi wao na kuguswa na kuunda kloridi amonia (NH 4 Cl (s)), glaze nyeupe mara nyingi kuonekana kwenye kioo. Kwa kuzingatia kwamba idadi ya moles ya kila gesi ambayo inakimbia ndani ya chumba ni sawa, ni nini shinikizo la sehemu ya juu ya HCl na NH 3 katika maabara kwenye joto la kawaida? (Kidokezo: Shinikizo la sehemu litakuwa sawa na lina thamani ya juu wakati wa usawa.)
S16.4.22
1.0 × 10 -8 atm. Hii ni shinikizo la juu la gesi chini ya hali zilizoelezwa.
Q16.4.23
Benzini inaweza kuandaliwa kutoka kwa acetylene. \(\ce{3C2H2}(g)⇌\ce{C6H6}(g)\). Kuamua mara kwa mara ya usawa saa 25 °C na saa 850 °C Je, mmenyuko wa pekee katika mojawapo ya joto hizi? Kwa nini asetilini yote haipatikani kama benzini?
Q16.4.24
Dioksidi ya kaboni hutengana ndani ya CO na O 2 kwenye joto la juu. Je, ni shinikizo la sehemu ya usawa wa oksijeni katika sampuli saa 1000 °C ambayo shinikizo la awali la CO 2 lilikuwa 1.15 atm?
\[x=\mathrm{1.29×10^{−5}\:atm}=P_{\ce{O2}}\]
Q16.4.25
Tetrachloride ya kaboni, kutengenezea muhimu ya viwanda, imeandaliwa na klorini ya methane saa 850 K.
\[\ce{CH4}(g)+\ce{4Cl2}(g)⟶\ce{CCl4}(g)+\ce{4HCl}(g)\]
Je, ni mara kwa mara ya usawa kwa mmenyuko wa 850 K? Je, chombo cha majibu kinahitajika kuwa joto au kilichopozwa ili kuweka joto la majibu mara kwa mara?
Q16.4.25B
Asidi ya Acetic, CH 3 CO 2 H, inaweza kuunda dimer, (CH 3 CO 2 H) 2, katika awamu ya gesi.
\[\ce{2CH3CO2H}(g)⟶\ce{(CH3CO2H)2}(g)\]
Dimer inafanyika pamoja na vifungo viwili vya hidrojeni na nguvu ya jumla ya 66.5 kJ kwa mole ya dimer.
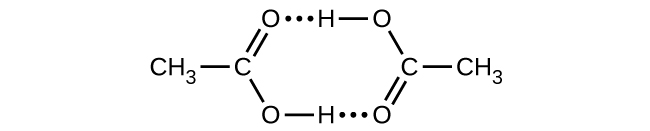
Katika 25 °C, mara kwa mara ya usawa kwa dimerization ni 1.3 × 10 3 (shinikizo katika atm). Je, ni Δ S° kwa mmenyuko?
S16.4.25B
-0.16 kJ
Q16.4.26
Asidi ya nitriki, HNO 3, inaweza kuandaliwa na mlolongo wa athari zifuatazo:
\[\ce{4NH3}(g)+\ce{5O2}(g)⟶\ce{4NO}(g)+\ce{6H2O}(g)\]
\[\ce{2NO}(g)+\ce{O2}(g)⟶\ce{2NO2}(g)\]
\[\ce{3NO2}(g)+\ce{H2O}(l)⟶\ce{2HNO3}(l)+\ce{NO}(g)\]
How much heat is evolved when 1 mol of NH3(g) is converted to HNO3(l)? Assume standard states at 25 °C.
Q16.4.27A
Determine ΔG for the following reactions.
(a) Antimony pentachloride decomposes at 448 °C. The reaction is:
\[\ce{SbCl5}(g)⟶\ce{SbCl3}(g)+\ce{Cl2}(g)\]
An equilibrium mixture in a 5.00 L flask at 448 °C contains 3.85 g of SbCl5, 9.14 g of SbCl3, and 2.84 g of Cl2.
Chlorine molecules dissociate according to this reaction:
\[\ce{Cl2}(g)⟶\ce{2Cl}(g)\]
1.00% of Cl2 molecules dissociate at 975 K and a pressure of 1.00 atm.
S16.4.27A
- (a) −22.1 kJ;
- 61.6 kJ/mol
Q16.4.27
Given that the \(ΔG^\circ_\ce{f}\) for Pb2+(aq) and Cl−(aq) is −24.3 kJ/mole and −131.2 kJ/mole respectively, determine the solubility product, Ksp, for PbCl2(s).
Q16.4.28
Determine the standard free energy change, \(ΔG^\circ_\ce{f}\), for the formation of S2−(aq) given that the \(ΔG^\circ_\ce{f}\) for Ag+(aq) and Ag2S(s) are 77.1 k/mole and −39.5 kJ/mole respectively, and the solubility product for Ag2S(s) is 8 × 10−51.
S16.4.28
90 kJ/mol
Q16.4.29
Determine the standard enthalpy change, entropy change, and free energy change for the conversion of diamond to graphite. Discuss the spontaneity of the conversion with respect to the enthalpy and entropy changes. Explain why diamond spontaneously changing into graphite is not observed.
Q16.4.30
The evaporation of one mole of water at 298 K has a standard free energy change of 8.58 kJ.
\[\ce{H2O}(l)⇌\ce{H2O}(g) \hspace{20px} ΔG^\circ_{298}=\mathrm{8.58\: kJ}\]
- (a) Is the evaporation of water under standard thermodynamic conditions spontaneous?
- Determine the equilibrium constant, KP, for this physical process.
- By calculating ∆G, determine if the evaporation of water at 298 K is spontaneous when the partial pressure of water, \(P_{\ce{H2O}}\), is 0.011 atm.
- If the evaporation of water were always nonspontaneous at room temperature, wet laundry would never dry when placed outside. In order for laundry to dry, what must be the value of \(P_{\ce{H2O}}\) in the air?
S16.4.30
(a) Under standard thermodynamic conditions, the evaporation is nonspontaneous; Kp = 0.031; The evaporation of water is spontaneous; \(P_{\ce{H2O}}\) must always be less than Kp or less than 0.031 atm. 0.031 atm represents air saturated with water vapor at 25 °C, or 100% humidity.
Q16.4.31
In glycolysis, the reaction of glucose (Glu) to form glucose-6-phosphate (G6P) requires ATP to be present as described by the following equation:
\[\mathrm{Glu + ATP ⟶ G6P + ADP} \hspace{20px} ΔG^\circ_{298}=\mathrm{−17\: kJ}\]
In this process, ATP becomes ADP summarized by the following equation:
\[\mathrm{ATP⟶ADP} \hspace{20px} ΔG^\circ_{298}=\mathrm{−30\: kJ}\]
Determine the standard free energy change for the following reaction, and explain why ATP is necessary to drive this process:
\[\mathrm{Glu⟶G6P} \hspace{20px} ΔG^\circ_{298}=\:?\]
Q16.4.32
One of the important reactions in the biochemical pathway glycolysis is the reaction of glucose-6-phosphate (G6P) to form fructose-6-phosphate (F6P):
\[\mathrm{G6P⇌F6P} \hspace{20px} ΔG^\circ_{298}=\mathrm{1.7\: kJ}\]
- (a) Is the reaction spontaneous or nonspontaneous under standard thermodynamic conditions?
- Standard thermodynamic conditions imply the concentrations of G6P and F6P to be 1 M, however, in a typical cell, they are not even close to these values. Calculate ΔG when the concentrations of G6P and F6P are 120 μM and 28 μM respectively, and discuss the spontaneity of the forward reaction under these conditions. Assume the temperature is 37 °C.
S16.4.32
(a) Nonspontaneous as \(ΔG^\circ_{298}>0\); \(ΔG^\circ_{298}=−RT\ln K,\) \(ΔG = 1.7×10^3 + \left(8.314 × 335 × \ln\dfrac{28}{128}\right) = \mathrm{−2.5\: kJ}\). The forward reaction to produce F6P is spontaneous under these conditions.
Q16.4.33
Without doing a numerical calculation, determine which of the following will reduce the free energy change for the reaction, that is, make it less positive or more negative, when the temperature is increased. Explain.
- (a) \(\ce{N2}(g)+\ce{3H2}(g)⟶\ce{2NH3}(g)\)
- \(\ce{HCl}(g)+\ce{NH3}(g)⟶\ce{NH4Cl}(s)\)
- \(\ce{(NH4)2Cr2O7}(s)⟶\ce{Cr2O3}(s)+\ce{4H2O}(g)+\ce{N2}(g)\)
- \(\ce{2Fe}(s)+\ce{3O2}(g)⟶\ce{Fe2O3}(s)\)
When ammonium chloride is added to water and stirred, it dissolves spontaneously and the resulting solution feels cold. Without doing any calculations, deduce the signs of ΔG, ΔH, and ΔS for this process, and justify your choices.
S16.4.33
ΔG is negative as the process is spontaneous. ΔH is positive as with the solution becoming cold, the dissolving must be endothermic. ΔS must be positive as this drives the process, and it is expected for the dissolution of any soluble ionic compound.
Q16.4.34
An important source of copper is from the copper ore, chalcocite, a form of copper(I) sulfide. When heated, the Cu2S decomposes to form copper and sulfur described by the following equation:
\[\ce{Cu2S}(s)⟶\ce{Cu}(s)+\ce{S}(s)\]
- (a) Determine \(ΔG^\circ_{298}\) for the decomposition of Cu2S(s).
- The reaction of sulfur with oxygen yields sulfur dioxide as the only product. Write an equation that describes this reaction, and determine \(ΔG^\circ_{298}\) for the process.
- The production of copper from chalcocite is performed by roasting the Cu2S in air to produce the Cu. By combining the equations from Parts (a) and (b), write the equation that describes the roasting of the chalcocite, and explain why coupling these reactions together makes for a more efficient process for the production of the copper.
Q16.4.35
What happens to \(ΔG^\circ_{298}\) (becomes more negative or more positive) for the following chemical reactions when the partial pressure of oxygen is increased?
- (a) \(\ce{S}(s)+\ce{O2}(g)⟶\ce{SO2}(g)\)
- \(\ce{2SO2}(g)+\ce{O2}(g)⟶\ce{SO3}(g)\)
- \(\ce{HgO}(s)⟶\ce{Hg}(l)+\ce{O2}(g)\)
S16.4.35
- (a) Increasing \(P_{\ce{O2}}\) will shift the equilibrium toward the products, which increases the value of K. \(ΔG^\circ_{298}\) therefore becomes more negative.
- Increasing \(P_{\ce{O2}}\) will shift the equilibrium toward the products, which increases the value of K. \(ΔG^\circ_{298}\) therefore becomes more negative.
- Increasing \(P_{\ce{O2}}\) will shift the equilibrium the reactants, which decreases the value of K. \(ΔG^\circ_{298}\) therefore becomes more positive.


