7.E: Kemikali Bonding na Masi jiometri (Mazoezi)
- Page ID
- 176641
7.1: Ionic Bonding
Q7.1.1
Je, cation inapata protoni kuunda chaji chanya au inapoteza elektroni?
S7.1.1
Protoni katika kiini hazibadilika wakati wa athari za kawaida za kemikali. Ni elektroni za nje tu zinazohamia. Mashtaka mazuri huunda wakati elektroni zinapotea.
Q7.1.2
Sulfate ya chuma (III) [Fe 2 (SO 4) 3] inajumuisha Fe 3+ na\(\ce{SO4^2-}\) ions. Eleza kwa nini sampuli ya sulfate ya chuma (III) haijafunguliwa.
Q7.1.3
Ni ipi kati ya atomi zifuatazo zitatarajiwa kuunda ions hasi katika misombo ya ioniki ya binary na ambayo itatarajiwa kuunda ions chanya: P, I, Mg, Cl, Katika, Cs, O, Pb, Co?
S7.1.3
P, I, Cl, na O bila kuunda anions kwa sababu wao ni nonmetals. Mg, Katika, Cs, Pb, na Co bila kuunda cations kwa sababu wao ni metali.
Q7.1.4
Ni ipi kati ya atomi zifuatazo zitatarajiwa kuunda ioni hasi katika misombo ya ioniki ya binary na ambayo itatarajiwa kuunda ions chanya: Br, Ca, Na, N, F, Al, Sn, S, Cd?
Q7.1.5
Kutabiri malipo juu ya ions monatomic sumu kutoka atomi zifuatazo katika misombo binary ionic:
- P
- Mg
- Al
- O
- Cl
- Cs
S7.1.5
P 3—; Mg 2+; Al 3+; The 2—; Cl —; Cs +
Q7.1.6
Kutabiri malipo juu ya ions monatomic sumu kutoka atomi zifuatazo katika misombo binary ionic:
- I
- Sr
- K
- N
- S
- Katika
S7.1.6
- Mimi -
- Sr 2+
- K +
- N 3-
- S 2-
- Katika 3+
Q7.1.7
Andika usanidi wa elektroni kwa kila ions zifuatazo:
- Kama 3—
- Mimi —
- Kuwa 2+
- Cd 2+
- O 2—
- Ga 3+
- Li +
- (h) N 3—
- (i) Sn 2+
- (j) Co 2+
- (k) Fe 2+
- (l) kama 3+
S7.1.7
[Ar] 4 s 2 3 d 10 4 p 6; [Kr] 4 d 10 5 s 2 5 p 6 1 s 2 [Kr] 4 d 10; [yeye] 2 s 2 2 p 6; [Ar] 3 d 10; 1 s 2 (h) [yeye] 2 s 2 p 6 (i) [Kr] 4 d 10 5 s 2 (j) [Ar] 3 d 7 (k) [Ar] 3 d 6, (l) [Ar] 3 d 10 4 s 2
Q7.1.8
Andika usanidi wa elektroni kwa ions za monatomiki zilizoundwa kutoka kwa mambo yafuatayo (ambayo huunda mkusanyiko mkubwa wa ions za monatomiki katika maji ya bahari):
- Cl
- Na
- Mg
- Ca
- K
- Br
- Sr
- (h) F
Q7.1.9
Andika usanidi kamili wa elektroni kwa kila atomi zifuatazo na kwa ion ya monatomiki iliyopatikana katika misombo ya ioniki ya binary iliyo na kipengele:
- Al
- Br
- Sr
- Li
- Kama
- S
S7.1.9
1 s 2 s 2 s 2 p 6 3 s 2 p 1; Al 3+: 1 s 2 s 2 s 2 p 6; 1 s 2; 2 s 2; s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 5; 1 s 2 s 2 p 6 , 3 s 2, 3 p 6, 3 d, 10, 4 s, 2, 4 s, 2, p, 6, 3 s; 2, 3 p 6, 3 d 10, 4 s 2, 4 p 6, 5 s 2;
Sr 2+: 1 s 2 s 2 s 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2; 4 p 6; 1 s 2; s 1;
Li +: 1 s 2; 1 s 2 s 2 s 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 3; 1 s 2 2 s 2 p 6 3 s 2 3 p 6 3 d 3 d 10 4 s 2 p 6; 1 s 2 s 2: 2 p 6, 3 s, 2, 3 p 4; 1 s 2, 2, p, 6, 3, p 6;
Q7.1.10
Kutoka kwa maandiko ya bidhaa kadhaa za kibiashara, jitayarisha orodha ya misombo sita ya ionic katika bidhaa. Kwa kila kiwanja, weka formula. (Unaweza haja ya kuangalia juu ya baadhi ya formula katika kumbukumbu kufaa.)
7.3: Kuunganishwa kwa uwiano
Kwa nini si sahihi kuzungumza juu ya molekuli ya NaCl imara?
NaCl ina ions discrete mpangilio katika kimiani kioo, si covalently bonded molekuli.
Ni habari gani unaweza kutumia kutabiri kama dhamana kati ya atomi mbili ni covalent au ionic?
Kutabiri ni ipi ya misombo yafuatayo ni ionic na ambayo ni covalent, kulingana na eneo la atomi zao za majimbo katika meza ya mara kwa mara:
- Cl 2 CO
- Hapana
- nCl 3
- Cobr 2
- K 2 S
- USHIRIKIANO
- CaF 2
- (h) HI
- (i) CaO
- (j) Ibr
- (k) CO 2
ionic: (b), (d), (e), (g), na (i); covalent: (a), (c), (f), (h), (j), na (k)
Eleza tofauti kati ya dhamana isiyo ya kawaida ya covalent, dhamana ya covalent ya polar, na dhamana ya ionic.
Kutoka nafasi yake katika meza ya mara kwa mara, onyesha ni atomi gani katika kila jozi ni zaidi ya electronegative:
- Bar au Cl
- N au O
- S au O
- P au S
- Si au N
- Ba au P
- N au K
Cl; O; O; S; N; P; N
Kutoka nafasi yake katika meza ya mara kwa mara, onyesha ni atomi gani katika kila jozi ni zaidi ya electronegative:
- N au P
- N au Ge
- S au F
- Cl au S
- H au C
- Se au P
- C au Si
Kutoka nafasi zao katika meza ya mara kwa mara, tengeneza atomi katika kila mfululizo wafuatayo ili kuongeza electronegativity:
- C, F, H, N, O
- Br, Cl, F, H, I
- KAMA, H, O, P, S
- All, H, Na, O, P
- Ba, H, N, O, Kama
H, C, N, O, F; H, I, Br, Cl, F; H, P, S, O, F; Na, Al, H, P, O; Ba, H, Kama, N, O
Kutoka nafasi zao katika meza ya mara kwa mara, tengeneza atomi katika kila mfululizo wafuatayo ili kuongeza electronegativity:
- Kama, H, N, P, Sb
- Cl, H, P, S, Si
- Br, Cl, Ge, H, Sr
- Ca, H, K, N, Si
- Cl, Cs, Ge, H, Sr
Ambayo atomi inaweza kushikamana na sulfuri ili kuzalisha chanya sehemu chaji juu ya atomi sulfuri?
N, O, F, na Cl
Ambayo ni dhamana ya polar zaidi?
- C—C
- C—H
- N—H
- O—H
- Se—H
Tambua dhamana zaidi ya polar katika kila jozi zifuatazo za vifungo:
- HF au HCl
- NO au CO
- SH au OH
- PCL au Scl
- CH au NH
- SO au PO
- CN au NN
HF; CO; OH; PCL; NH; PO; CN
Ni ipi kati ya molekuli zifuatazo au ions zenye vifungo vya polar?
- YA 3
- S 8
- \(\ce{O2^2-}\)
- \(\ce{NO3-}\)
- CO 2
- H 2 S
- \(\ce{BH4-}\)
7.4: Alama za Lewis na Miundo
Q7.4.1
Andika alama Lewis kwa kila moja ya ions zifuatazo:
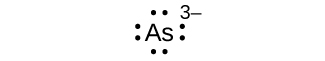
- Kama 3—
- Mimi —
- Kuwa 2+
- O 2—
- Ga 3+
- Li +
- N 3—
S7.4.1
elektroni nane:
elektroni nane:

hakuna elektroni
Kuwa 2+;
elektroni nane:
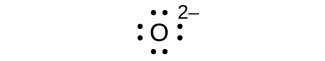
hakuna elektroni
Ga 3+;
hakuna elektroni
Li +;
elektroni nane:
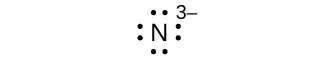
Q7.4.2
Ions nyingi za monatomiki zinapatikana katika maji ya bahari, ikiwa ni pamoja na ions zilizoundwa kutoka kwenye orodha ya vipengele vifuatavyo. Andika alama za Lewis kwa ions za monatomiki zilizoundwa kutoka kwa vipengele vifuatavyo:
- Cl
- Na
- Mg
- Ca
- K
- Br
- Sr
- F
Q7.4.3
Andika alama za Lewis za ions katika kila moja ya misombo ya ionic ifuatayo na alama za Lewis za atomi ambazo hutengenezwa:
- MGs
- All 2 Ya 3
- gacl 3
- K 2 O
- Li 3 N
- KF
(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)
 ;
;
(f)

Katika miundo ya Lewis iliyoorodheshwa hapa, M na X huwakilisha vipengele mbalimbali katika kipindi cha tatu cha meza ya mara kwa mara. Andika fomu ya kila kiwanja kwa kutumia alama za kemikali za kila kipengele:
(a)

(b)

(c)

(d)

Andika muundo wa Lewis kwa molekuli ya diatomic P 2, aina isiyo imara ya fosforasi iliyopatikana katika mvuke ya fosforasi ya juu.

Andika miundo Lewis kwa yafuatayo:
- H 2
- HbR
- PCL 3
- SF 2
- H 2 CH 2
- HNH
- H 2 CNH
- (h) HAPANA —
- (i) N 2
- (j) CO
- (k) CN -
Andika miundo Lewis kwa yafuatayo:
- O 2
- H 2 CO
- SAF 3
- ClNo
- SiCl 4
- H 3 O +
- \(\ce{NH4+}\)
- (h) \(\ce{BF4-}\)
- (i) HCCH
- (j) ClCN
- (k) \(\ce{C2^2+}\)
(a)

In this case, the Lewis structure is inadequate to depict the fact that experimental studies have shown two unpaired electrons in each oxygen molecule.
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)
 ;
;
(f)
 ;
;
(g)
 ;
;
(h)
 ;
;
(i)
 ;
;
(j)
 ;
;
(k)

Andika miundo Lewis kwa yafuatayo:
- cLF 3
- PCL 5
- BF 3
- \(\ce{PF6-}\)
Write Lewis structures for the following:
- SeF6
- XeF4
- \(\ce{SeCl3+}\)
- Cl2BBCl2 (contains a B–B bond)
SeF6:
 ;
;
XeF4:
 ;
;
\(\ce{SeCl3+}\):
 ;
;
Cl 2 BBCl 2:

Andika Lewis miundo kwa:
- \(\ce{PO4^3-}\)
- \(\ce{ICl4-}\)
- \(\ce{SO3^2-}\)
- HONO
Correct the following statement: “The bonds in solid PbCl2 are ionic; the bond in a HCl molecule is covalent. Thus, all of the valence electrons in PbCl2 are located on the Cl– ions, and all of the valence electrons in a HCl molecule are shared between the H and Cl atoms.”
Two valence electrons per Pb atom are transferred to Cl atoms; the resulting Pb2+ ion has a 6s2 valence shell configuration. Two of the valence electrons in the HCl molecule are shared, and the other six are located on the Cl atom as lone pairs of electrons.
Write Lewis structures for the following molecules or ions:
- SbH3
- XeF2
- Se8 (a cyclic molecule with a ring of eight Se atoms)
Methanol, H3COH, is used as the fuel in some race cars. Ethanol, C2H5OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO2 and H2O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.

Many planets in our solar system contain organic chemicals including methane (CH4) and traces of ethylene (C2H4), ethane (C2H6), propyne (H3CCCH), and diacetylene (HCCCCH). Write the Lewis structures for each of these molecules.
Carbon tetrachloride was formerly used in fire extinguishers for electrical fires. It is no longer used for this purpose because of the formation of the toxic gas phosgene, Cl2CO. Write the Lewis structures for carbon tetrachloride and phosgene.

Tambua atomi zinazohusiana na kila moja ya maandamano ya elektroni yafuatayo. Kisha, andika alama ya Lewis kwa ioni ya kawaida inayotokana na kila atomu:
- 1 s 2 2 s 2 p 5
- 1 s 2 2 s 2 p 6 3 s 2
- 1 s 2 2 s 2 p 6 3 s 2 3 s 2 p 6 4 s 2 3 d 10
- 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 4
- 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 1
Mpangilio wa atomi katika molekuli kadhaa muhimu za kibiolojia hutolewa hapa. Kukamilisha miundo ya Lewis ya molekuli hizi kwa kuongeza vifungo vingi na jozi pekee. Usiongeze atomi yoyote zaidi.
serine ya amino asidi:

urea:

asidi ya piruvic:

uracil:

asidi kaboni:

(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)

Kiwanja kilicho na molekuli ya molar ya takriban 28 g/mol kina kaboni 85.7% na hidrojeni 14.3% kwa wingi. Andika muundo wa Lewis kwa molekuli ya kiwanja.
Kiwanja kilicho na molekuli ya molar ya 42 g/mol kina 85.7% kaboni na 14.3% hidrojeni kwa wingi. Andika muundo wa Lewis kwa molekuli ya kiwanja.

Mipango miwili ya atomi inawezekana kwa kiwanja kilicho na masi ya molar ya takriban 45 g/mol ambayo ina 52.2% C, 13.1% H, na 34.7% O kwa wingi. Andika miundo Lewis kwa molekuli mbili.
Je, ni vifungo vya moja, mara mbili, na vitatu vinavyofanana? Wanatofautiaje?
Kila dhamana inajumuisha ugawaji wa elektroni kati ya atomi. Elektroni mbili zinashirikiwa katika dhamana moja; elektroni nne zinashirikiwa katika dhamana mbili; na elektroni sita zinashirikiwa katika dhamana tatu.
7.5: Mashtaka rasmi na Resonance
Andika fomu za resonance zinazoelezea usambazaji wa elektroni katika kila moja ya molekuli hizi au ions.
- seleniamu dioksidi, SEO
- nitrati ioni,\(\ce{NO3-}\)
- nitric acid, HNO3 (N is bonded to an OH group and two O atoms)
- benzene, C6H6:

ioni ya formate:

Andika fomu za resonance zinazoelezea usambazaji wa elektroni katika kila moja ya molekuli hizi au ions.
- dioksidi sulfuri, SO 2
- ioni ya kaboni,\(\ce{CO3^2-}\)
- hydrogen carbonate ion, \(\ce{HCO3-}\) (C is bonded to an OH group and two O atoms)
- pyridine:

allyl ion:

(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)

Andika aina za resonance za ozoni, O 3, sehemu ya anga ya juu ambayo inalinda Dunia kutokana na mionzi ya ultraviolet.
Nitriti ya sodiamu, ambayo imetumiwa kuhifadhi bakoni na nyama nyingine, ni kiwanja cha ionic. Andika aina za resonance za ion ya nitriti,\(\ce{NO2-}\).

Kwa upande wa vifungo sasa, kueleza kwa nini asidi asetiki, CH 3 CO 2 H, ina aina mbili tofauti ya vifungo kaboni-oksijeni, ambapo ion acetate, sumu kwa hasara ya ioni hidrojeni kutoka asidi asetiki, ina aina moja tu ya carbon-oksijeni dhamana. Miundo ya mifupa ya aina hizi inavyoonyeshwa:

Andika miundo Lewis kwa yafuatayo, na ni pamoja na miundo resonance inapofaa. Eleza ambayo ina dhamana yenye nguvu ya kaboni-oksijeni.
- CO 2
- USHIRIKIANO
(a)

(b)

CO ina dhamana yenye nguvu ya kaboni-oksijeni kwa sababu kuna dhamana ya mara tatu inayojiunga na C na O. CO 2 ina vifungo viwili.
Toothpastes zenye carbonate ya hidrojeni ya sodiamu (bicarbonate ya sodiamu) na peroxide ya Andika miundo ya Lewis kwa ioni ya carbonate ya hidrojeni na molekuli ya peroxide ya hidrojeni, na
Kuamua malipo rasmi ya kila kipengele katika zifuatazo:
- HCl
- CF 4
- PCL 3
- PF 5
H: 0, Cl: 0; C: 0, F: 0; P: 0, Cl 0; P: 0, F: 0
Kuamua malipo rasmi ya kila kipengele katika zifuatazo:
- H 3 O +
- \(\ce{SO4^2-}\)
- NH3
- \(\ce{O2^2-}\)
- H2O2
Calculate the formal charge of chlorine in the molecules Cl2, BeCl2, and ClF5.
Cl in Cl2: 0; Cl in BeCl2: 0; Cl in ClF5: 0
Calculate the formal charge of each element in the following compounds and ions:
- F2CO
- NO–
- \(\ce{BF4-}\)
- \(\ce{SnCl3-}\)
- H2CCH2
- ClF3
- SeF6
- (h) \(\ce{PO4^3-}\)
Draw all possible resonance structures for each of these compounds. Determine the formal charge on each atom in each of the resonance structures:
- O3
- SO2
- \(\ce{NO2-}\)
- \(\ce{NO3-}\)
 ;
;
(b)
 ;
;
(c)
![[Two Lewis structures are shown, with brackets surrounding each with a superscripted negative sign and a double ended arrow in between. The left structure shows a nitrogen atom with one lone pair of electrons single bonded to an oxygen atom with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. The symbols and numbers below this structure read “open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, negative 1, close parenthesis. The right structure appears as a mirror image of the left and the symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis.]](http://cnx.org/resources/1fdade826754e56f88dd8009bac80fa91af0bebc/CNX_Chem_07_04_Exercis12c_img.jpg) ;
;
(d)
![[Tatu Lewis miundo ni umeonyesha, na mabano jirani kila na superscripted ishara hasi na mara mbili kumalizika mshale katika kati. Muundo wa kushoto unaonyesha atomi ya nitrojeni moja iliyounganishwa na atomi mbili za oksijeni, kila mmoja akiwa na jozi tatu za elektroni na mara mbili zimeunganishwa na atomu ya oksijeni yenye jozi mbili za elektroni. Single bonded oksijeni atomi ni kinachoitwa, kutoka juu ya muundo na kwenda clockwise, “wazi mabano, hasi 1, karibu mabano, wazi mabano, chanya 1, karibu mabano”. Ishara na namba zilizo chini ya muundo huu zinasoma “wazi mabano, 0, mabano ya karibu, mabano ya wazi, hasi 1, mabano ya karibu. muundo wa kati inaonyesha nitrojeni atomi moja Bonded kwa atomi mbili oksijeni, kila mmoja na jozi tatu lone ya elektroni, moja ambayo ni kinachoitwa “wazi mabano, chanya 1, karibu mabano” na mara mbili Bonded kwa atomi oksijeni na jozi mbili lone ya elektroni kinachoitwa “wazi mabano, 0, karibu mabano”. Ishara na namba zilizo chini ya muundo huu zinasoma “wazi mabano, hasi 1, mabano ya karibu, mabano ya wazi, hasi 1, mabano ya karibu. Muundo sahihi unaonyesha atomi ya nitrojeni moja iliyounganishwa na atomi mbili za oksijeni, kila mmoja akiwa na jozi tatu za elektroni na mara mbili zimeunganishwa na atomu ya oksijeni yenye jozi mbili za elektroni. Moja ya atomi moja Bonded oksijeni ni kinachoitwa, “wazi mabano, hasi 1, karibu mabano wakati mara mbili Bonded oksijeni ni kinachoitwa, “wazi mabano, chanya 1, karibu mabano”. Ishara na namba zilizo chini ya muundo huu zinasoma “mabano ya wazi, hasi 1, mabano ya karibu” na “mabano ya wazi, 0, mabano ya karibu”.]](http://cnx.org/resources/263fd2cd3c8d574474c489b5bb2f41b94cd81ae5/CNX_Chem_07_04_Exercis12d_img.jpg)
Kulingana na masuala rasmi ya malipo, ni ipi ya yafuatayo ingekuwa mpangilio sahihi wa atomi katika kloridi ya nitrosyl: ClNO au ClOn?
Kulingana na masuala rasmi ya malipo, ni ipi kati ya yafuatayo ingekuwa mpangilio sahihi wa atomi katika asidi ya hypochlorous: HoCl au OClH?
HoCl
Kulingana na masuala rasmi ya malipo, ni ipi kati ya yafuatayo ingekuwa mpangilio sahihi wa atomi katika dioksidi sulfuri: OSO au SOO?
Chora muundo wa hydroxylamine, H 3 NO, na uwape mashtaka rasmi; angalia muundo. Je muundo halisi sambamba na mashtaka rasmi?
muundo kwamba anatoa zero mashtaka rasmi ni sambamba na muundo halisi:

Iodini huunda mfululizo wa fluorides (iliyoorodheshwa hapa). Andika miundo ya Lewis kwa kila moja ya misombo minne na ueleze malipo rasmi ya atomi ya iodini katika kila molekuli:
- KAMA
- KAMA 3
- KAMA 5
- KAMA 7
Andika muundo wa Lewis na formula ya kemikali ya kiwanja na molekuli ya molar ya 70 g/mol ambayo ina 19.7% ya nitrojeni na fluorini 80.3% kwa wingi, na kuamua malipo rasmi ya atomi katika kiwanja hiki.
NF 3;

Ni ipi kati ya miundo ifuatayo tunatarajia kwa asidi ya nitrous? Kuamua mashtaka rasmi:

Asidi ya sulfuriki ni kemikali ya viwanda inayozalishwa kwa wingi mkubwa duniani kote. Takriban paundi bilioni 90 huzalishwa kila mwaka nchini Marekani pekee. Andika muundo wa Lewis kwa asidi ya sulfuriki, H 2 SO 4, ambayo ina atomi mbili za oksijeni na makundi mawili ya OH yaliyounganishwa na sulfuri.

7.6: Nguvu za vifungo vya Ionic na Covalent
Ni dhamana gani katika kila jozi zifuatazo za vifungo ni nguvu zaidi?
- C—C au\(\mathrm{C=C}\)
- C–N or \(\mathrm{C≡N}\)
- \(\mathrm{C≡O}\) or \(\mathrm{C=O}\)
- H–F or H–Cl
- C–H or O–H
- C–N or C–O
Using the bond energies in Table, determine the approximate enthalpy change for each of the following reactions:
- \(\ce{H2}(g)+\ce{Br2}(g)⟶\ce{2HBr}(g)\)
- \(\ce{CH4}(g)+\ce{I2}(g)⟶\ce{CH3I}(g)+\ce{HI}(g)\)
- (c) \(\ce{C2H4}(g)+\ce{3O2}(g)⟶\ce{2CO2}(g)+\ce{2H2O}(g)\)
- −114 kJ;
- 30 kJ;
- (c) −1055 kJ
Using the bond energies in Table, determine the approximate enthalpy change for each of the following reactions:
- \(\ce{Cl2}(g)+\ce{3F2}(g)⟶\ce{2ClF3}(g)\)
- \(\mathrm{H_2C=CH_2}(g)+\ce{H2}(g)⟶\ce{H3CCH3}(g)\)
- (c) \(\ce{2C2H6}(g)+\ce{7O2}(g)⟶\ce{4CO2}(g)+\ce{6H2O}(g)\)
When a molecule can form two different structures, the structure with the stronger bonds is usually the more stable form. Use bond energies to predict the correct structure of the hydroxylamine molecule:

The greater bond energy is in the figure on the left. It is the more stable form.
How does the bond energy of HCldiffer from the standard enthalpy of formation of HCl(g)?
Using the standard enthalpy of formation data in Appendix G, show how the standard enthalpy of formation of HCl(g) can be used to determine the bond energy.
\(\ce{HCl}(g)⟶\dfrac{1}{2}\ce{H2}(g)+\dfrac{1}{2}\ce{Cl2}(g)\hspace{20px}ΔH^\circ_1=−ΔH^\circ_{\ce f[\ce{HCl}(g)]}\\
\dfrac{1}{2}\ce{H2}(g)⟶\ce{H}(g)\hspace{105px}ΔH^\circ_2=ΔH^\circ_{\ce f[\ce H(g)]}\\
\underline{\dfrac{1}{2}\ce{Cl2}(g)⟶\ce{Cl}(g)\hspace{99px}ΔH^\circ_3=ΔH^\circ_{\ce f[\ce{Cl}(g)]}}\\
\ce{HCl}(g)⟶\ce{H}(g)+\ce{Cl}(g)\hspace{58px}ΔH^\circ_{298}=ΔH^\circ_1+ΔH^\circ_2+ΔH^\circ_3\)
\(\begin{align}
D_\ce{HCl}=ΔH^\circ_{298}&=ΔH^\circ_{\ce f[\ce{HCl}(g)]}+ΔH^\circ_{\ce f[\ce H(g)]}+ΔH^\circ_{\ce f[\ce{Cl}(g)]}\\
&=\mathrm{−(−92.307\:kJ)+217.97\:kJ+121.3\:kJ}\\
&=\mathrm{431.6\:kJ}
\end{align}\)
Using the standard enthalpy of formation data in Appendix G, calculate the bond energy of the carbon-sulfur double bond in CS2.
Using the standard enthalpy of formation data in Appendix G, determine which bond is stronger: the S–F bond in SF4(g) or in SF6(g)?
The S–F bond in SF4 is stronger.
Using the standard enthalpy of formation data in Appendix G, determine which bond is stronger: the P–Cl bond in PCl3(g) or in PCl5(g)?
Complete the following Lewis structure by adding bonds (not atoms), and then indicate the longest bond:


The C–C single bonds are longest.
Use the bond energy to calculate an approximate value of ΔH for the following reaction. Which is the more stable form of FNO2?

Use principles of atomic structure to answer each of the following:1
- The radius of the Ca atom is 197 pm; the radius of the Ca2+ ion is 99 pm. Account for the difference.
- The lattice energy of CaO(s) is –3460 kJ/mol; the lattice energy of K2O is –2240 kJ/mol. Account for the difference.
- (c) Given these ionization values, explain the difference between Ca and K with regard to their first and second ionization energies.
| Element | First Ionization Energy (kJ/mol) | Second Ionization Energy (kJ/mol) |
|---|---|---|
| K | 419 | 3050 |
| Ca | 590 | 1140 |
The first ionization energy of Mg is 738 kJ/mol and that of Al is 578 kJ/mol. Account for this difference.
When two electrons are removed from the valence shell, the Ca radius loses the outermost energy level and reverts to the lower n = 3 level, which is much smaller in radius. The +2 charge on calcium pulls the oxygen much closer compared with K, thereby increasing the lattice energy relative to a less charged ion. (c) Removal of the 4s electron in Ca requires more energy than removal of the 4s electron in K because of the stronger attraction of the nucleus and the extra energy required to break the pairing of the electrons. The second ionization energy for K requires that an electron be removed from a lower energy level, where the attraction is much stronger from the nucleus for the electron. In addition, energy is required to unpair two electrons in a full orbital. For Ca, the second ionization potential requires removing only a lone electron in the exposed outer energy level. In Al, the removed electron is relatively unprotected and unpaired in a p orbital. The higher energy for Mg mainly reflects the unpairing of the 2s electron.
The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 200.8 pm. NaF crystallizes in the same structure as LiF but with a Na–F distance of 231 pm. Which of the following values most closely approximates the lattice energy of NaF: 510, 890, 1023, 1175, or 4090 kJ/mol? Explain your choice.
For which of the following substances is the least energy required to convert one mole of the solid into separate ions?
- MgO
- SrO
- (c) KF
- CsF
- MgF2
(d)
The reaction of a metal, M, with a halogen, X2, proceeds by an exothermic reaction as indicated by this equation: \(\ce{M}(s)+\ce{X2}(g)⟶\ce{MX2}(s)\). For each of the following, indicate which option will make the reaction more exothermic. Explain your answers.
- a large radius vs. a small radius for M+2
- a high ionization energy vs. a low ionization energy for M
- (c) an increasing bond energy for the halogen
- a decreasing electron affinity for the halogen
- an increasing size of the anion formed by the halogen
The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 201 pm. MgO crystallizes in the same structure as LiF but with a Mg–O distance of 205 pm. Which of the following values most closely approximates the lattice energy of MgO: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ/mol, or 4008 kJ/mol? Explain your choice.
4008 kJ/mol; both ions in MgO have twice the charge of the ions in LiF; the bond length is very similar and both have the same structure; a quadrupling of the energy is expected based on the equation for lattice energy
Which compound in each of the following pairs has the larger lattice energy? Note: Mg2+ and Li+ have similar radii; O2– and F– have similar radii. Explain your choices.
- MgO or MgSe
- LiF or MgO
- (c) Li2O or LiCl
- Li2Se or MgO
Which compound in each of the following pairs has the larger lattice energy? Note: Ba2+ and
K+ have similar radii; S2– and Cl– have similar radii. Explain your choices.
- K2O or Na2O
- K2S or BaS
- (c) KCl or BaS
- BaS or BaCl2
Na2O; Na+ has a smaller radius than K+; BaS; Ba has a larger charge than K; (c) BaS; Ba and S have larger charges; BaS; S has a larger charge
Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- MgO
- SrO
- (c) KF
- CsF
- MgF2
Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
- K2S
- K2O
- (c) CaS
- Cs2S
- CaO
(e)
The lattice energy of KF is 794 kJ/mol, and the interionic distance is 269 pm. The Na–F
distance in NaF, which has the same structure as KF, is 231 pm. Which of the following values is the closest approximation of the lattice energy of NaF: 682 kJ/mol, 794 kJ/mol, 924 kJ/mol, 1588 kJ/mol, or 3175 kJ/mol? Explain your answer.
7.7: Molecular Structure and Polarity
Explain why the HOH molecule is bent, whereas the HBeH molecule is linear.
The placement of the two sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting HOH molecule is bent. The HBeH molecule (in which Be has only two electrons to bond with the two electrons from the hydrogens) must have the electron pairs as far from one another as possible and is therefore linear.
What feature of a Lewis structure can be used to tell if a molecule’s (or ion’s) electron-pair geometry and molecular structure will be identical?
Explain the difference between electron-pair geometry and molecular structure.
Space must be provided for each pair of electrons whether they are in a bond or are present as lone pairs. Electron-pair geometry considers the placement of all electrons. Molecular structure considers only the bonding-pair geometry.
Why is the H–N–H angle in NH3 smaller than the H–C–H bond angle in CH4? Why is the H–N–H angle in \(\ce{NH4+}\) identical to the H–C–H bond angle in CH4?
Explain how a molecule that contains polar bonds can be nonpolar.
As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar.
As a general rule, MXn molecules (where M represents a central atom and X represents terminal atoms; n = 2 – 5) are polar if there is one or more lone pairs of electrons on M. NH3 (M = N, X = H, n = 3) is an example. There are two molecular structures with lone pairs that are exceptions to this rule. What are they?
Predict the electron pair geometry and the molecular structure of each of the following molecules or ions:
- SF6
- PCl5
- (c) BeH2
- \(\ce{CH3+}\)
- Both the electron geometry and the molecular structure are octahedral.
- Both the electron geometry and the molecular structure are trigonal bipyramid.
- (c) Both the electron geometry and the molecular structure are linear.
- Both the electron geometry and the molecular structure are trigonal planar.
Identify the electron pair geometry and the molecular structure of each of the following molecules or ions:
- \(\ce{IF6+}\)
- CF4
- (c) BF3
- \(\ce{SiF5-}\)
- BeCl2
What are the electron-pair geometry and the molecular structure of each of the following molecules or ions?
- ClF5
- \(\ce{ClO2-}\)
- (c) \(\ce{TeCl4^2-}\)
- PCl3
- SeF4
- \(\ce{PH2-}\)
electron-pair geometry: octahedral, molecular structure: square pyramidal; electron-pair geometry: tetrahedral, molecular structure: bent; (c) electron-pair geometry: octahedral, molecular structure: square planar; electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal; electron-pair geometry: trigonal bypyramidal, molecular structure: seesaw; electron-pair geometry: tetrahedral, molecular structure: bent (109°)
Predict the electron pair geometry and the molecular structure of each of the following ions:
- H3O+
- \(\ce{PCl4-}\)
- (c) \(\ce{SnCl3-}\)
- \(\ce{BrCl4-}\)
- ICl3
- XeF4
- (g) SF2
Identify the electron pair geometry and the molecular structure of each of the following molecules:
- ClNO (N is the central atom)
- CS2
- (c) Cl2CO (C is the central atom)
- Cl2SO (S is the central atom)
- SO2F2 (S is the central atom)
- XeO2F2 (Xe is the central atom)
- (g) \(\ce{ClOF2+}\) (Cl is the central atom)
electron-pair geometry: trigonal planar, molecular structure: bent (120°); electron-pair geometry: linear, molecular structure: linear; (c) electron-pair geometry: trigonal planar, molecular structure: trigonal planar; electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal; electron-pair geometry: tetrahedral, molecular structure: tetrahedral; electron-pair geometry: trigonal bipyramidal, molecular structure: seesaw; (g) electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
Predict the electron pair geometry and the molecular structure of each of the following:
- IOF5 (I is the central atom)
- POCl3 (P is the central atom)
- (c) Cl2SeO (Se is the central atom)
- ClSO+ (S is the central atom)
- F2SO (S is the central atom)
- \(\ce{NO2-}\)
- (g) \(\ce{SiO4^4-}\)
Which of the following molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
- ClF5
- \(\ce{ClO2-}\)
- (c) \(\ce{TeCl4^2-}\)
- PCl3
- SeF4
- \(\ce{PH2-}\)
- (g) XeF2
All of these molecules and ions contain polar bonds. Only ClF5, \(\ce{ClO2-}\), PCl3, SeF4, and \(\ce{PH2-}\) have dipole moments.
Which of the molecules and ions in Exercise contain polar bonds? Which of these molecules and ions have dipole moments?
- H3O+
- \(\ce{PCl4-}\)
- (c) \(\ce{SnCl3-}\)
- \(\ce{BrCl4-}\)
- ICl3
- XeF4
- (g) SF2
Which of the following molecules have dipole moments?
- CS2
- SeS2
- (c) CCl2F2
- PCl3 (P is the central atom)
- ClNO (N is the central atom)
SeS2, CCl2F2, PCl3, and ClNO all have dipole moments.
Identify the molecules with a dipole moment:
- SF4
- CF4
- (c) Cl2CCBr2
- CH3Cl
- H2CO
The molecule XF3 has a dipole moment. Is X boron or phosphorus?
P
The molecule XCl2 has a dipole moment. Is X beryllium or sulfur?
Is the Cl2BBCl2 molecule polar or nonpolar?
nonpolar
There are three possible structures for PCl2F3 with phosphorus as the central atom. Draw them and discuss how measurements of dipole moments could help distinguish among them.
Describe the molecular structure around the indicated atom or atoms:
- the sulfur atom in sulfuric acid, H2SO4 [(HO)2SO2]
- the chlorine atom in chloric acid, HClO3 [HOClO2]
- (c) the oxygen atom in hydrogen peroxide, HOOH
- the nitrogen atom in nitric acid, HNO3 [HONO2]
- the oxygen atom in the OH group in nitric acid, HNO3 [HONO2]
- the central oxygen atom in the ozone molecule, O3
- (g) each of the carbon atoms in propyne, CH3CCH
- (h) the carbon atom in Freon, CCl2F2
- (i) each of the carbon atoms in allene, H2CCCH2
tetrahedral; trigonal pyramidal; (c) bent (109°); trigonal planar; bent (109°); bent (109°); (g) CH3CCH tetrahedral, CH3CCH linear; (h) tetrahedral; (i) H2CCCH2 linear; H2CCCH2 trigonal planar
Draw the Lewis structures and predict the shape of each compound or ion:
- CO2
- \(\ce{NO2-}\)
- (c) SO3
- \(\ce{SO3^2-}\)
A molecule with the formula AB2, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion for each shape.

Molekuli yenye formula AB 3, ambayo A na B huwakilisha atomi tofauti, inaweza kuwa na moja ya maumbo matatu tofauti. Mchoro na jina maumbo matatu tofauti ambayo molekuli hii inaweza kuwa. Kutoa mfano wa molekuli au ioni ambayo ina kila umbo.
Chora Lewis elektroni dot miundo kwa molekuli hizi, ikiwa ni pamoja na miundo resonance inapofaa:
- \(\ce{CS3^2-}\)
- CS 2
- (c) CS
kutabiri maumbo Masi kwa\(\ce{CS3^2-}\) na CS 2 na kueleza jinsi aliwasili katika utabiri wako
(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
\(\ce{CS3^2-}\) includes three regions of electron density (all are bonds with no lone pairs); the shape is trigonal planar; CS2 has only two regions of electron density (all bonds with no lone pairs); the shape is linear
What is the molecular structure of the stable form of FNO2? (N is the central atom.)
A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen. What is its molecular structure?
The Lewis structure is made from three units, but the atoms must be rearranged:

Tumia simulation kufanya mazoezi yafuatayo kwa molekuli mbili za atomi:
- Kurekebisha thamani electronegativity hivyo dhamana dipole ni akizungumzia kuelekea B. kisha kuamua nini maadili electronegativity lazima kubadili dipole ili pointi kuelekea A.
- Kwa malipo mazuri ya A, tembea shamba la umeme na ueleze kinachotokea.
- (c) Kwa malipo madogo ya sehemu ya A, tembea shamba la umeme na ueleze kinachotokea.
- Weka upya wote, na kisha kwa malipo makubwa ya sehemu ya A, tembea shamba la umeme na ueleze kinachotokea.
Tumia simulation kufanya mazoezi yafuatayo kwa molekuli halisi. Unaweza kuhitaji mzunguko wa molekuli katika vipimo vitatu ili kuona dipoles fulani.
- Mchoro dipoles dhamana na dipole Masi (kama ipo) kwa O 3. Eleza uchunguzi wako.
- Angalia dipoles dhamana kwa NH 3. Tumia dipoles hizi kutabiri kama N au H ni zaidi ya electronegative.
- (c) Kutabiri kama kuna lazima iwe na dipole ya Masi kwa NH 3 na, ikiwa ni hivyo, katika mwelekeo gani utaelezea. Angalia sanduku la dipole la Masi ili kupima hypothesis yako.
Dipole ya Masi inaelekea mbali na atomi za hidrojeni.
Tumia simulator ya Shape ya Molekuli ili kujenga molekuli. Kuanzia na chembe ya kati, bonyeza dhamana mara mbili ili kuongeza dhamana moja mara mbili. Kisha kuongeza dhamana moja na jozi moja pekee. Mzunguko molekuli kuchunguza jiometri kamili. Jina la kikundi cha elektroni jiometri na muundo wa Masi na kutabiri pembe ya dhamana. Kisha bofya masanduku ya hundi chini na kulia ya simulator ili uangalie majibu yako.
Kutumia simulator Molekuli Shape kuchunguza molekuli halisi. Kwenye kichupo cha Real Molekuli, chagua H 2 O. Badilisha kati ya modes “halisi” na “mfano”. Eleza tofauti iliyozingatiwa.
Miundo ni sawa sana. Katika hali ya mfano, kila kikundi cha elektroni kinachukua kiasi sawa cha nafasi, hivyo angle ya dhamana inaonyeshwa kama 109.5°. Katika hali “halisi”, jozi pekee ni kubwa, na kusababisha hidrojeni kusisitizwa. Hii inasababisha angle ndogo ya 104.5°.
Kutumia simulator Molekuli Shape kuchunguza molekuli halisi. Kwenye tab ya Molekuli halisi, chagua “mode” mode na S 2 O. ni angle ya dhamana ya mfano gani? Eleza kama angle “halisi” ya dhamana inapaswa kuwa kubwa au ndogo kuliko angle bora ya mfano.


