6.4: Muundo wa umeme wa Atomi (Configurations Electron)
- Page ID
- 176159
- Kupata alitabiri configurations ardhi hali elektroni ya atomi
- Kutambua na kueleza tofauti kwa usanidi wa elektroni uliotabiriwa kwa atomi na ions
- Eleza mipangilio ya elektroni na uainishaji wa kipengele katika meza ya mara kwa mara
Baada ya kuanzisha misingi ya muundo wa atomiki na mechanics ya quantum, tunaweza kutumia ufahamu wetu wa idadi ya quantum kuamua jinsi orbitals ya atomiki yanahusiana na kila mmoja. Hii inatuwezesha kuamua ni vipi ambavyo vinashikiliwa na elektroni katika kila atomi. Mpangilio maalum wa elektroni katika orbitali ya atomu huamua mali nyingi za kemikali za atomi hiyo.
Nguvu za Orbital na muundo wa Atomiki
Nishati ya orbitals ya atomiki huongezeka kama idadi kuu ya quantum,\(n\), huongezeka. Katika atomi yoyote yenye elektroni mbili au zaidi, kupinduliwa kati ya elektroni hufanya nguvu za subshells na maadili\(l\) tofauti ya tofauti ili nishati ya orbitali iongezeke ndani ya shell ili s <p <d <f Kielelezo \(\PageIndex{1}\)inaonyesha jinsi hizi mwenendo mbili katika kuongeza nishati kuhusiana. The 1 s orbital chini ya mchoro ni orbital na elektroni ya nishati ya chini. Nishati huongezeka kama sisi hoja juu ya 2 s na kisha 2 p, 3 s, na 3 p orbitals, kuonyesha kwamba ongezeko n thamani ina ushawishi zaidi juu ya nishati ya kuongeza l thamani kwa atomi ndogo. Hata hivyo, ruwaza hii haina kushikilia kwa atomi kubwa. Ya 3 d orbital ni ya juu katika nishati kuliko 4 s orbital. Uingiliano huo unaendelea kutokea mara kwa mara kama sisi hoja juu ya chati.
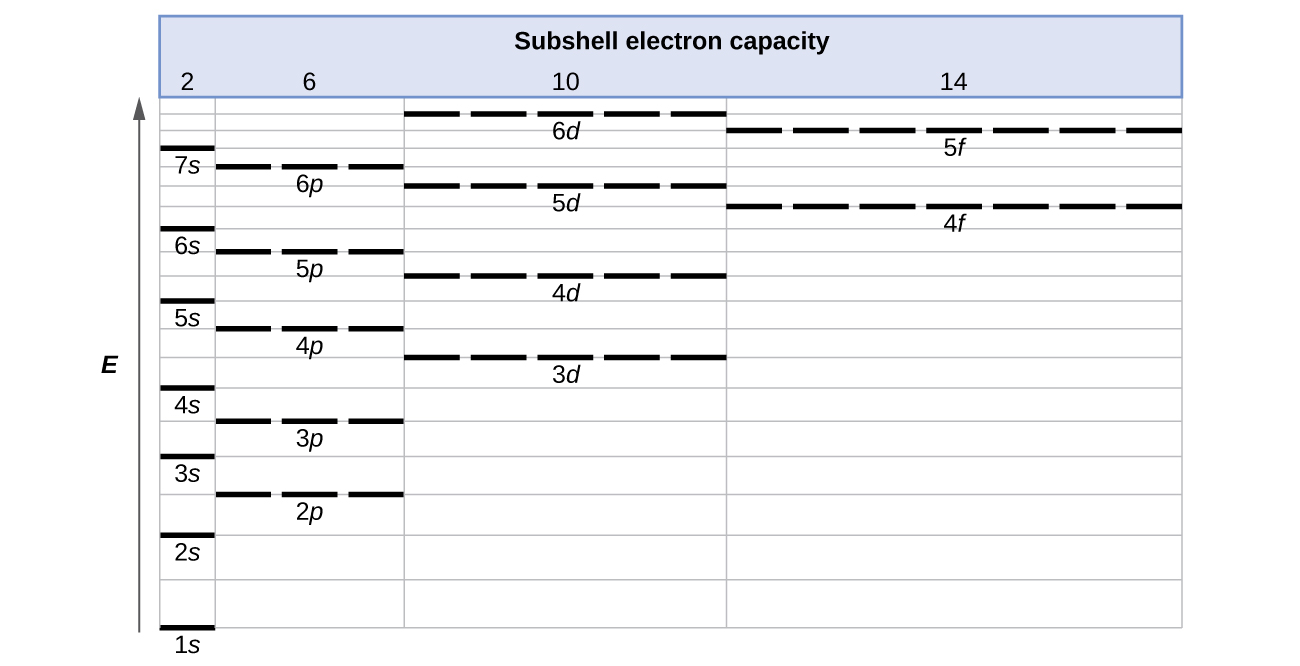
Electroni katika atomi za mfululizo kwenye meza ya mara kwa mara huwa na kujaza orbitali za nishati ya chini kwanza. Kwa hiyo, wanafunzi wengi wanaona kuwa ni kuchanganya kwamba, kwa mfano, orbitals 5 p kujaza mara moja baada ya 4 d, na mara moja kabla ya 6 s. Utaratibu wa kujaza unategemea matokeo ya majaribio yaliyozingatiwa, na imethibitishwa na mahesabu ya kinadharia. Kama idadi kuu ya quantum, n, huongezeka, ukubwa wa ongezeko la orbital na elektroni hutumia muda zaidi mbali na kiini. Hivyo, kivutio kwa kiini ni dhaifu na nishati inayohusishwa na orbital ni ya juu (chini imetulia). Lakini hii sio athari pekee tunayopaswa kuzingatia. Ndani ya kila shell, kama thamani ya l inavyoongezeka, elektroni hazipatikani (maana kuna wiani mdogo wa elektroni unaopatikana karibu na kiini), kwa utaratibu s> p> d> f. Elektroni zilizo karibu na kiini hurudisha kidogo elektroni zilizo mbali zaidi, zikizuia vivutio vingi vya elektroni-kiini kidogo (kumbuka kuwa elektroni zote zina mashtaka -1, lakini viini vina mashtaka + Z). Jambo hili linaitwa shielding na litajadiliwa kwa undani zaidi katika sehemu inayofuata. Electroni katika orbitals kwamba uzoefu zaidi shielding ni chini imetulia na hivyo juu katika nishati. Kwa orbitals ndogo (1 s kupitia 3 p), ongezeko la nishati kutokana na n ni muhimu zaidi kuliko ongezeko kutokana na l; hata hivyo, kwa orbitals kubwa mwenendo miwili ni sawa na hauwezi kutabiriwa tu. Tutazungumzia mbinu za kukumbuka utaratibu uliozingatiwa.
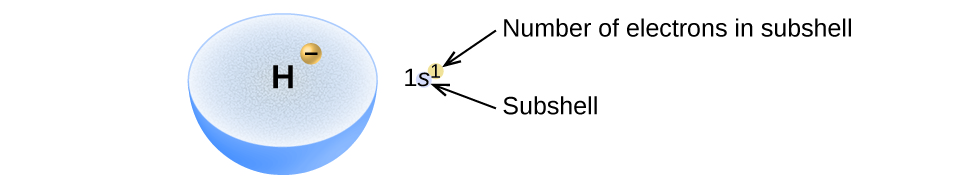
Mpangilio wa elektroni katika orbitals ya atomi huitwa usanidi wa elektroni wa atomi. Tunaelezea usanidi wa elektroni na ishara ambayo ina vipande vitatu vya habari (Kielelezo\(\PageIndex{2}\)):
- Idadi ya shell kuu ya quantum, n,
- Barua ambayo inaashiria aina ya orbital (subshell, l), na
- Nambari ya superscript ambayo inataja idadi ya elektroni katika sehemu hiyo ndogo.
Kwa mfano, nukuu 2 p 4 (soma “mbili—p—nne”) inaonyesha elektroni nne katika p subshell (l = 1) yenye namba kuu ya quantum (n) ya 2. Nukuu 3 d 8 (soma “tatu-d—nane”) inaonyesha elektroni nane katika sehemu ndogo ya d (yaani, l = 2) ya shell kuu ambayo n = 3.
Kanuni ya Aufbau
Kuamua usanidi wa elektroni kwa atomi yoyote fulani, tunaweza “kujenga” miundo kwa utaratibu wa namba za atomiki. Kuanzia na hidrojeni, na kuendelea katika vipindi vya meza ya mara kwa mara, tunaongeza protoni moja kwa wakati kwa kiini na elektroni moja kwa subshell sahihi mpaka tumeelezea usanidi wa elektroni wa vipengele vyote. Utaratibu huu unaitwa kanuni ya Aufbau, kutoka neno la Kijerumani Aufbau (“kujenga”). Kila elektroni iliyoongezwa inachukua subshell ya nishati ya chini zaidi inapatikana (kwa utaratibu ulioonyeshwa kwenye Kielelezo\(\PageIndex{3}\)), chini ya mapungufu yaliyowekwa na namba za quantum zilizoruhusiwa kulingana na kanuni ya kutengwa kwa Pauli. Electroni huingia subshells za juu-nishati tu baada ya subshells za chini za nishati zimejazwa kwa uwezo. Kielelezo\(\PageIndex{3}\) unaeleza njia ya jadi kukumbuka ili kujaza kwa orbitals atomiki.
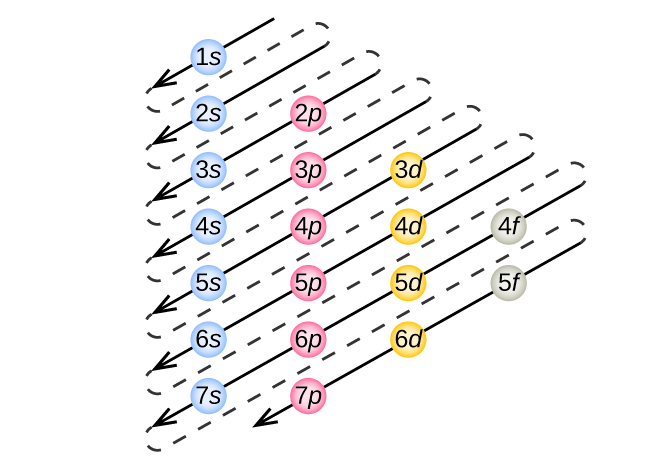
Kwa kuwa mpangilio wa meza ya mara kwa mara unategemea usanidi wa elektroni, Kielelezo\(\PageIndex{4}\) hutoa njia mbadala ya kuamua usanidi wa elektroni. Utaratibu wa kujaza huanza tu kwenye hidrojeni na hujumuisha kila subshell unapoendelea kuongezeka kwa utaratibu wa Z. Kwa mfano, baada ya kujaza 3 p kuzuia hadi Ar, tunaona orbital itakuwa 4s (K, Ca), ikifuatiwa na orbitals 3 d.
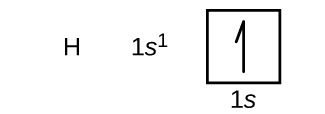
Sasa tutajenga usanidi wa elektroni wa hali ya ardhi na mchoro wa orbital kwa uteuzi wa atomi katika vipindi vya kwanza na vya pili vya meza ya mara kwa mara. Michoro ya Orbital ni uwakilishi wa picha ya usanidi wa elektroni, kuonyesha orbitals binafsi na mpangilio wa pairing wa elektroni. Tunaanza na atomi moja ya hidrojeni (namba atomiki 1), ambayo ina proton moja na elektroni moja. Akizungumzia ama Kielelezo\(\PageIndex{3}\) au\(\PageIndex{4}\), tunataka kutarajia kupata elektroni katika orbital 1 s. Kwa mkataba,\(m_s=+\dfrac{1}{2}\) thamani ni kawaida kujazwa kwanza. Configuration ya elektroni na mchoro wa orbital ni:
Kufuatia hidrojeni ni heli ya gesi yenye heshima, ambayo ina namba atomia ya 2. Atomu ya heliamu ina protoni mbili na elektroni mbili. Electroni ya kwanza ina namba nne za quantum sawa na elektroni ya atomu ya hidrojeni (n = 1, l = 0, m l = 0,\(m_s=+\dfrac{1}{2}\)). Electroni ya pili pia inakwenda katika orbital ya 1 s na inajaza orbital hiyo. Electron ya pili ina idadi sawa ya n, l, na m l quantum, lakini lazima iwe na idadi tofauti ya quantum ya spin,\(m_s=−\dfrac{1}{2}\). Hii ni kwa mujibu wa kanuni ya kutengwa kwa Pauli: Hakuna elektroni mbili katika atomu moja zinaweza kuwa na seti sawa ya namba nne za quantum. Kwa michoro orbital, hii inamaanisha mishale miwili inakwenda katika kila sanduku (inayowakilisha elektroni mbili katika kila orbital) na mishale lazima ielekeze kwa njia tofauti (inayowakilisha spins zilizounganishwa). Configuration ya elektroni na mchoro orbital ya heliamu ni:
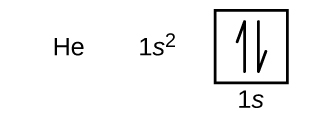
Ganda la n = 1 limejaa kabisa atomu ya heliamu.
Atomu inayofuata ni lithiamu ya metali ya alkali yenye namba atomia ya 3. Electroni mbili za kwanza katika lithiamu zinajaza orbital ya 1 s na zina seti sawa za namba nne za quantum kama elektroni mbili katika heliamu. Electron iliyobaki inapaswa kuchukua orbital ya nishati ya chini zaidi, 2 s orbital (Kielelezo\(\PageIndex{3}\) au\(\PageIndex{4}\)). Hivyo, usanidi wa elektroni na mchoro wa orbital wa lithiamu ni:
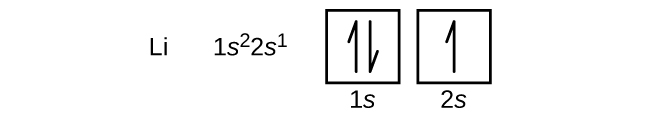
Atomu ya berili ya chuma ya alkali ya dunia, yenye namba atomia ya 4, ina protoni nne katika kiini na elektroni nne zinazozunguka kiini. Electroni ya nne inajaza nafasi iliyobaki katika orbital ya 2 s.
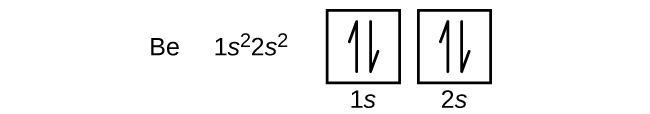
Atomu ya boroni (namba atomia 5) ina elektroni tano. Hifadhi ya n = 1 imejaa elektroni mbili na elektroni tatu zitachukua shell n = 2. Kwa sababu subshell yoyote inaweza kuwa na elektroni mbili tu, elektroni ya tano inapaswa kuchukua ngazi ya pili ya nishati, ambayo itakuwa 2 p orbital. Kuna tatu zilizopungua 2 p orbitals (m l = -1, 0, +1) na elektroni inaweza kuchukua mojawapo ya orbitals hizi p. Wakati wa kuchora michoro orbital, sisi ni pamoja na masanduku tupu kuonyesha orbitals yoyote tupu katika subshell sawa kwamba sisi ni kujaza.
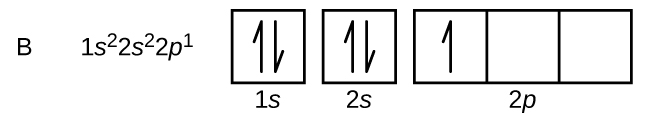
Kaboni (namba atomia 6) ina elektroni sita. Nne kati yao kujaza orbitals 1 s na 2 s. Electroni mbili zilizobaki zinachukua sehemu ya 2 p. Sasa tuna uchaguzi wa kujaza moja ya orbitals 2 p na pairing elektroni au kuacha elektroni unpaired katika mbili tofauti, lakini degenerate, p orbitals. Orbitals ni kujazwa kama ilivyoelezwa na utawala wa Hund: Configuration ya chini ya nishati kwa atomi na elektroni ndani ya seti ya orbitals degenerate ni kwamba kuwa na idadi kubwa ya elektroni unpaired. Hivyo, elektroni mbili katika carbon 2 p orbitals na idadi sawa n, l, na m s quantum na tofauti katika idadi yao m l quantum (kulingana na kanuni ya kutengwa Pauli). Configuration ya elektroni na mchoro orbital kwa kaboni ni:
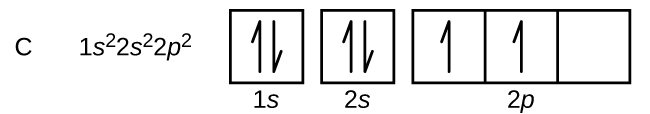
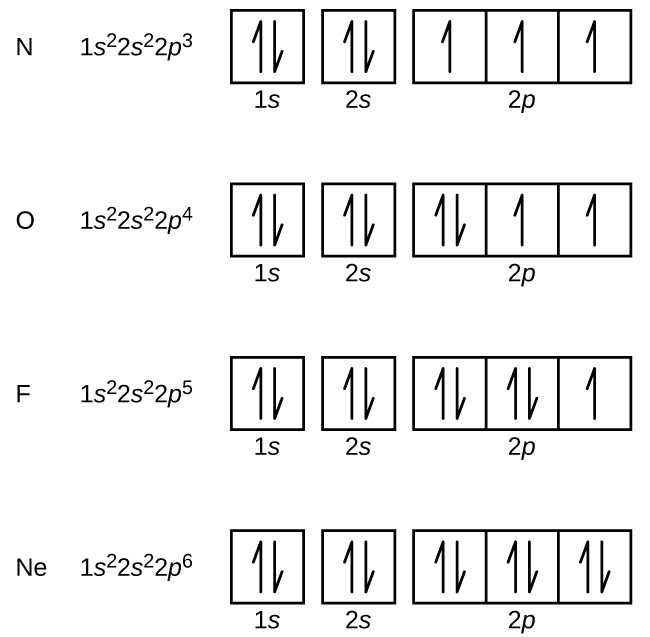
Nitrojeni (atomiki namba 7) hujaza subshells 1 na 2 s na ina elektroni moja katika kila moja ya orbitals tatu 2 p, kwa mujibu wa utawala wa Hund. Elektroni hizi tatu zina spins zisizoharibika. Oksijeni (namba atomia 8) ina jozi ya elektroni katika mojawapo ya orbitali 2 p (elektroni zina spins kinyume) na elektroni moja katika kila moja ya zile mbili nyingine. Fluorini (namba atomiki 9) ina moja tu ya 2 p orbital iliyo na elektroni isiyoharibika. Electroni zote katika neon ya gesi yenye heshima (namba ya atomiki 10) zimeunganishwa, na orbitals zote katika n = 1 na n = 2 shells zinajazwa. Configurations elektroni na michoro orbital ya mambo haya manne ni:
Sodiamu ya metali ya alkali (namba atomia 11) ina elektroni moja zaidi kuliko atomi ya neon. Electron hii inapaswa kuingia kwenye subshell ya chini ya nishati inapatikana, 3 s orbital, kutoa 1 s 2 s 2 s 2 p 6 3 s 1 usanidi. Electroni zinazotumia shell ya nje orbital (s) (thamani ya juu ya n) huitwa elektroni za valence, na wale wanaomiliki orbitals ya ndani ya shell huitwa elektroni za msingi (Kielelezo\ PageIndex5\ PageIndex5). Kwa kuwa makombora ya elektroni ya msingi yanahusiana na usanidi wa elektroni wa gesi yenye heshima, tunaweza kufupisha usanidi wa elektroni kwa kuandika gesi yenye heshima inayofanana na usanidi wa msingi wa elektroni, pamoja na elektroni za valence katika muundo uliofupishwa. Kwa mfano wetu wa sodiamu, ishara [Ne] inawakilisha elektroni za msingi, (1 s 2 s 2 s 2 p 6) na usanidi wetu uliofupishwa au uliofupishwa ni [Ne] 3 s 1 .

Vilevile, usanidi uliofupishwa wa lithiamu unaweza kuwakilishwa kama [He] 2 s 1, ambapo [Yeye] inawakilisha usanidi wa atomu ya heliamu, ambayo inafanana na ile ya ganda la ndani lililojazwa la lithiamu. Kuandika maandamano kwa njia hii inasisitiza kufanana kwa maandalizi ya lithiamu na sodiamu. Atomi zote mbili, ambazo ziko katika familia ya chuma ya alkali, zina elektroni moja tu katika subshell ya valence nje ya seti iliyojaa ya shells za ndani.
\[\ce{Li:[He]}\,2s^1\\ \ce{Na:[Ne]}\,3s^1 \nonumber \]
Magnesiamu ya chuma ya alkali ya ardhi (namba atomia 12), na elektroni zake 12 katika usanidi wa [Ne] 3 s 2, ni sawa na beryllium ya familia yake, [Yeye] 2 s 2. Atomi zote mbili zina shell iliyojaa nje ya maganda yao ya ndani yaliyojaa. Aluminium (namba atomia 13), yenye elektroni 13 na usanidi wa elektroni [Ne] 3 s 2 3 p 1, ni sawa na familia yake boroni, [Yeye] 2 s 2 2 p 1.
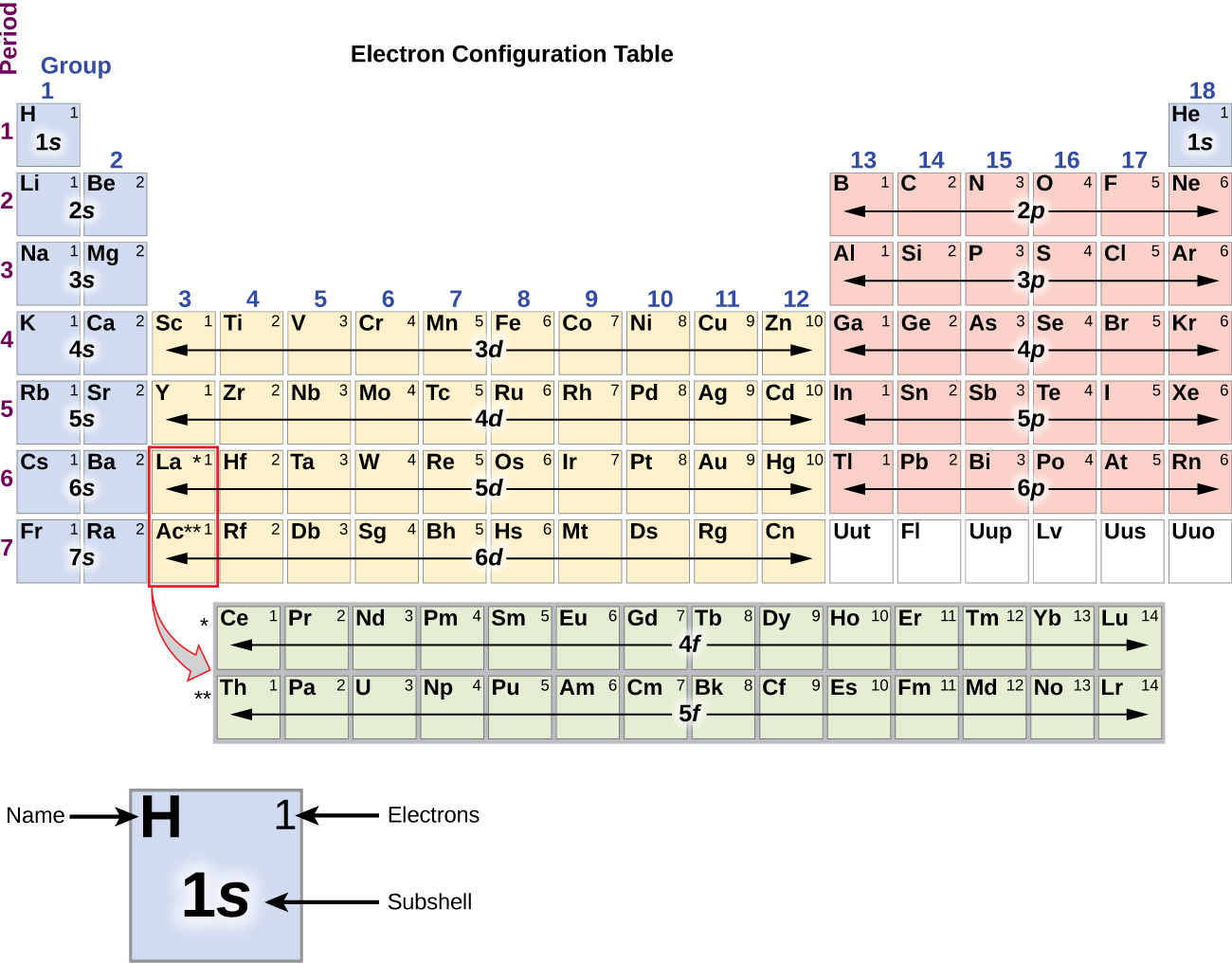
Mchanganyiko wa elektroni wa silicon (elektroni 14), fosforasi (elektroni 15), sulfuri (elektroni 16), klorini (elektroni 17), na Argon (elektroni 18) ni sawa katika usanidi wa elektroni wa maganda yao ya nje kwa wanachama wa familia zao zinazofanana kaboni, nitrojeni, oksijeni, fluorini, na neon, kwa mtiririko huo, isipokuwa kwamba idadi kuu ya quantum ya shell ya nje ya mambo nzito imeongezeka kwa moja hadi n = 3. Kielelezo\(\PageIndex{6}\) inaonyesha nishati ya chini, au ardhi hali, elektroni Configuration kwa mambo haya kama vile kwamba kwa atomi ya kila moja ya mambo inayojulikana.
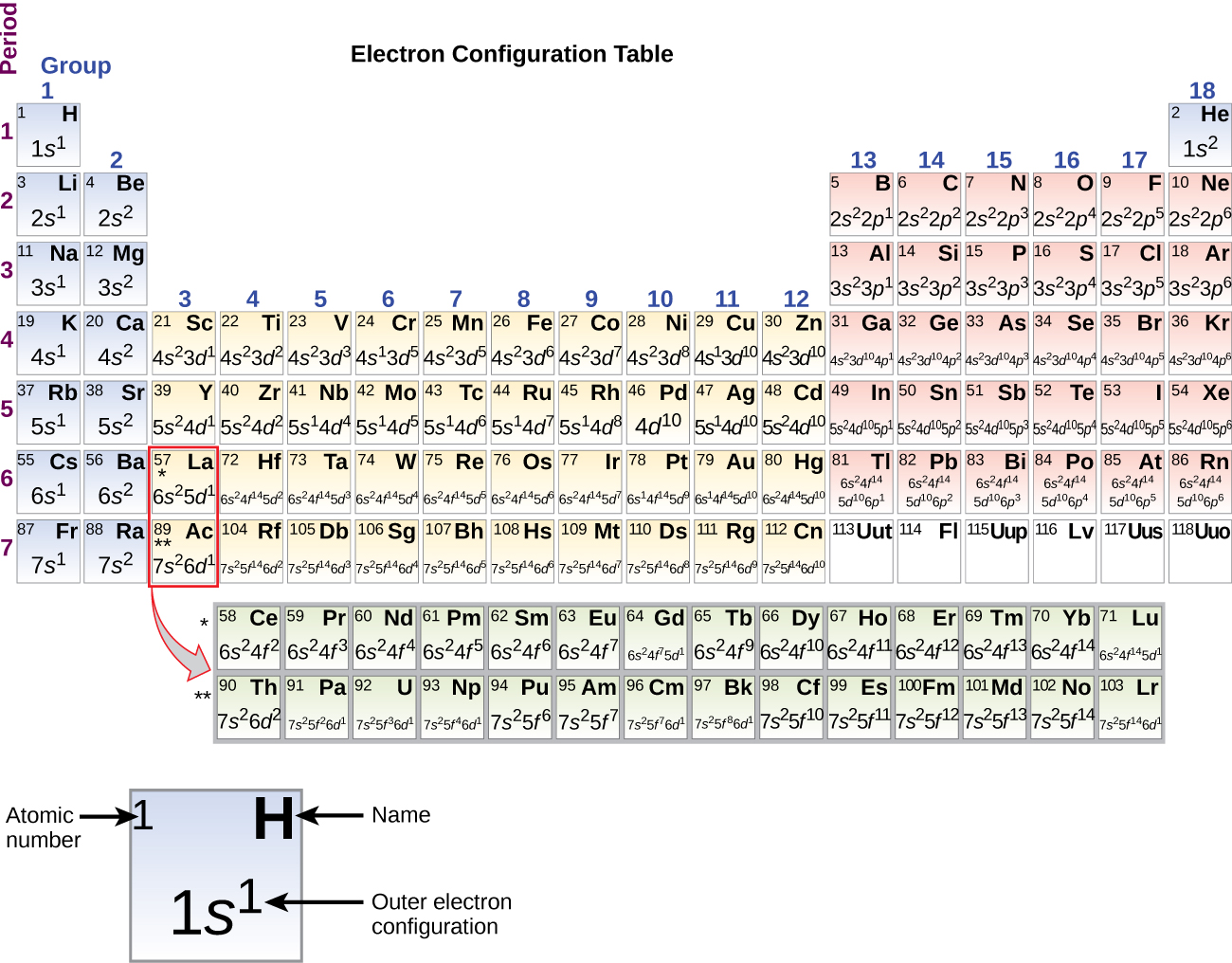
Wakati sisi kuja kipengele ijayo katika meza ya mara kwa mara, alkali chuma potasiamu (atomiki namba 19), tunaweza kutarajia kwamba tunataka kuanza kuongeza elektroni kwa subshell 3 d. Hata hivyo, kemikali zote zilizopo na ushahidi wa kimwili unaonyesha kwamba potasiamu ni kama lithiamu na sodiamu, na kwamba elektroni inayofuata haiongezwi kwa kiwango cha d 3 lakini, badala yake, imeongezwa kwa kiwango cha 4 s (Kielelezo\(\PageIndex{3}\) au\(\PageIndex{4}\)). Kama ilivyojadiliwa hapo awali, 3 d orbital bila nodes radial ni ya juu katika nishati kwa sababu ni chini ya kupenya na zaidi shielded kutoka kiini kuliko 4 s, ambayo ina nodes tatu radial. Hivyo, potasiamu ina usanidi wa elektroni wa [Ar] 4 s 1. Kwa hiyo, potasiamu inafanana na Li na Na katika usanidi wake wa shell ya valence. Elektroni inayofuata inaongezwa ili kukamilisha sehemu ndogo ya 4 s na kalsiamu ina usanidi wa elektroni wa [Ar] 4 s 2. Hii inatoa kalsiamu usanidi wa elektroni wa nje unaofanana na ule wa beryllium na magnesiamu.
Kuanzia na scandium ya chuma ya mpito (namba ya atomiki 21), elektroni za ziada zinaongezwa kwa mfululizo kwa subshell ya 3 d. Subshell hii imejaa uwezo wake na elektroni 10 (kumbuka kwamba kwa l = 2 [d orbitals], kuna 2 l + 1 = 5 maadili ya m l, maana yake ni kwamba kuna tano d orbitals ambayo ina uwezo wa pamoja wa elektroni 10). Subshell ya 4 p inajaza ijayo. Kumbuka kuwa kwa mfululizo wa vipengele vitatu, scandium (Sc) kupitia shaba (Cu), yttrium (Y) kupitia fedha (Ag), na lutetiamu (Lu) kupitia dhahabu (Au), jumla ya elektroni 10 d huongezwa mfululizo kwenye shell (n — 1) karibu na ganda la n ili kuleta hiyo (n — 1 ) shell kutoka elektroni 8 hadi 18. Kwa mfululizo mbili, lanthanum (La) kupitia lutetium (Lu) na actinium (Ac) kupitia lawrencium (Lr), 14 f elektroni (l = 3, 2 l + 1 = 7 m l maadili; hivyo, orbitals saba zilizo na uwezo wa pamoja wa elektroni 14) zinaongezwa kwa mfululizo kwa ( n — 2) ganda la kuleta ganda hilo kutoka elektroni 18 hadi jumla ya elektroni 32.
Configuration ya elektroni na mchoro orbital kwa atomi ya fosforasi ni nini? Nambari nne za quantum za elektroni ya mwisho zinaongezwa nini?
Suluhisho
Idadi ya atomiki ya fosforasi ni 15. Hivyo, atomi ya fosforasi ina elektroni 15. Utaratibu wa kujaza viwango vya nishati ni 1 s, 2 s, 2 p, 3 s, 3 p, 4 s,. Electroni 15 za atomi ya fosforasi zitajaza hadi 3 p orbital, ambayo itakuwa na elektroni tatu:
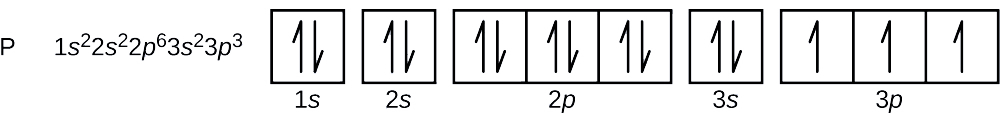
Elektroni ya mwisho imeongezwa ni elektroni ya 3 p. Kwa hiyo, n = 3 na, kwa p -aina orbital, l = 1. Thamani m l inaweza kuwa —1, 0, au +1. Orbitals tatu p ni degenerate, hivyo yoyote ya maadili haya m l ni sahihi. Kwa elektroni unpaired, mkataba inateua thamani ya\(+\dfrac{1}{2}\) for the spin quantum number; thus, \(m_s=+\dfrac{1}{2}\).
Identify the atoms from the electron configurations given:
- [Ar]4s23d5
- [Kr]5s24d105p6
- Answer a
-
Mn
- Answer b
-
Xe
The periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals that are shown in Figure \(\PageIndex{3}\) or \(\PageIndex{4}\). For instance, the electron configurations of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among others, are not those we would expect. In general, such exceptions involve subshells with very similar energy, and small effects can lead to changes in the order of filling.
In the case of Cr and Cu, we find that half-filled and completely filled subshells apparently represent conditions of preferred stability. This stability is such that an electron shifts from the 4s into the 3d orbital to gain the extra stability of a half-filled 3d subshell (in Cr) or a filled 3d subshell (in Cu). Other exceptions also occur. For example, niobium (Nb, atomic number 41) is predicted to have the electron configuration [Kr]5s24d3. Experimentally, we observe that its ground-state electron configuration is actually [Kr]5s14d4. We can rationalize this observation by saying that the electron–electron repulsions experienced by pairing the electrons in the 5s orbital are larger than the gap in energy between the 5s and 4d orbitals. There is no simple method to predict the exceptions for atoms where the magnitude of the repulsions between electrons is greater than the small differences in energy between subshells.
Electron Configurations and the Periodic Table
As described earlier, the periodic table arranges atoms based on increasing atomic number so that elements with the same chemical properties recur periodically. When their electron configurations are added to the table (Figure \(\PageIndex{6}\)), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. The outer electrons have the highest energy of the electrons in an atom and are more easily lost or shared than the core electrons. Valence electrons are also the determining factor in some physical properties of the elements.
Elements in any one group (or column) have the same number of valence electrons; the alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals beryllium and magnesium each have two, and the halogens fluorine and chlorine each have seven valence electrons. The similarity in chemical properties among elements of the same group occurs because they have the same number of valence electrons. It is the loss, gain, or sharing of valence electrons that defines how elements react.
It is important to remember that the periodic table was developed on the basis of the chemical behavior of the elements, well before any idea of their atomic structure was available. Now we can understand why the periodic table has the arrangement it has—the arrangement puts elements whose atoms have the same number of valence electrons in the same group. This arrangement is emphasized in Figure \(\PageIndex{6}\), which shows in periodic-table form the electron configuration of the last subshell to be filled by the Aufbau principle. The colored sections of Figure \(\PageIndex{6}\) show the three categories of elements classified by the orbitals being filled: main group, transition, and inner transition elements. These classifications determine which orbitals are counted in the valence shell, or highest energy level orbitals of an atom.
- Main group elements (sometimes called representative elements) are those in which the last electron added enters an s or a p orbital in the outermost shell, shown in blue and red in Figure \(\PageIndex{6}\). This category includes all the nonmetallic elements, as well as many metals and the intermediate semimetallic elements. The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s23d104p1, which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons.
- Transition elements or transition metals. These are metallic elements in which the last electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and (n – 1) d electrons. The official IUPAC definition of transition elements specifies those with partially filled d orbitals. Thus, the elements with completely filled orbitals (Zn, Cd, Hg, as well as Cu, Ag, and Au in Figure \(\PageIndex{6}\)) are not technically transition elements. However, the term is frequently used to refer to the entire d block (colored yellow in Figure \(\PageIndex{6}\)), and we will adopt this usage in this textbook.
- Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure \(\PageIndex{6}\). The valence shells of the inner transition elements consist of the (n – 2)f, the (n – 1)d, and the ns subshells. There are two inner transition series:
- The lanthanide series: lanthanide (La) through lutetium (Lu)
- The actinide series: actinide (Ac) through lawrencium (Lr)
Lanthanum and actinium, because of their similarities to the other members of the series, are included and used to name the series, even though they are transition metals with no f electrons.
Electron Configurations of Ions
We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a parent atom. For main group elements, the electrons that were added last are the first electrons removed. For transition metals and inner transition metals, however, electrons in the s orbital are easier to remove than the d or f electrons, and so the highest ns electrons are lost, and then the (n – 1)d or (n – 2)f electrons are removed. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. The added electrons fill in the order predicted by the Aufbau principle.
What is the electron configuration and orbital diagram of:
- Na+
- P3–
- Al2+
- Fe2+
- Sm3+
Solution
First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable.
Next, determine whether an electron is gained or lost. Remember electrons are negatively charged, so ions with a positive charge have lost an electron. For main group elements, the last orbital gains or loses the electron. For transition metals, the last s orbital loses an electron before the d orbitals.
- Na: 1s22s22p63s1. Sodium cation loses one electron, so Na+: 1s22s22p63s1 = Na+: 1s22s22p6.
- P: 1s22s22p63s23p3. Phosphorus trianion gains three electrons, so P3−: 1s22s22p63s23p6.
- Al: 1s22s22p63s23p1. Aluminum dication loses two electrons Al2+: 1s22s22p63s23p1 = Al2+: 1s22s22p63s1.
- Fe: 1s22s22p63s23p64s23d6. Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe2+: 1s22s22p63s23p64s23d6 = 1s22s22p63s23p63d6.
- Sm: 1s22s22p63s23p64s23d104p65s24d105p66s24f6. Samarium trication loses three electrons. The first two will be lost from the 6s orbital, and the final one is removed from the 4f orbital. Sm3+: 1s22s22p63s23p64s23d104p65s24d105p66s24f6 = 1s22s22p63s23p64s23d104p65s24d105p64f5.
- Which ion with a +2 charge has the electron configuration 1s22s22p63s23p63d104s24p64d5?
- Which ion with a +3 charge has this configuration?
- Answer a
-
Tc2+
- Answer b
-
Ru3+
Summary
The relative energy of the subshells determine the order in which atomic orbitals are filled (1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on). Electron configurations and orbital diagrams can be determined by applying the Pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and Hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals).
Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. There are some exceptions to the predicted filling order, particularly when half-filled or completely filled orbitals can be formed. The periodic table can be divided into three categories based on the orbital in which the last electron to be added is placed: main group elements (s and p orbitals), transition elements (d orbitals), and inner transition elements (f orbitals).
Glossary
- Aufbau principle
- procedure in which the electron configuration of the elements is determined by “building” them in order of atomic numbers, adding one proton to the nucleus and one electron to the proper subshell at a time
- core electron
- electron in an atom that occupies the orbitals of the inner shells
- electron configuration
- electronic structure of an atom in its ground state given as a listing of the orbitals occupied by the electrons
- Hund’s rule
- every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin
- orbital diagram
- pictorial representation of the electron configuration showing each orbital as a box and each electron as an arrow
- valence electrons
- electrons in the outermost or valence shell (highest value of n) of a ground-state atom; determine how an element reacts
- valence shell
- outermost shell of electrons in a ground-state atom; for main group elements, the orbitals with the highest n level (s and p subshells) are in the valence shell, while for transition metals, the highest energy s and d subshells make up the valence shell and for inner transition elements, the highest s, d, and f subshells are included


