10.3: 控制细胞周期
- Page ID
- 202737
培养技能
- 了解细胞周期是如何由细胞内部和外部机制控制的
- 解释三个内部控制检查点是如何发生在 G 1 末尾、G 2 /M 过渡时以及中期阶段的
- 描述通过正负调节控制细胞周期的分子
细胞周期的长度变化很大,即使在单个生物体的细胞内也是如此。 在人类中,细胞更新频率从早期胚胎发育的几个小时到上皮细胞的平均两到五天,再到皮质神经元或心肌细胞等特殊细胞在 G 0 中度过的整个人生中。 细胞在细胞周期的每个阶段所花费的时间也有所不同。 当快速分裂的哺乳动物细胞在培养物中(在最佳生长条件下在体外)生长时,周期长约为24小时。 在以 24 小时细胞周期快速分裂的人体细胞中,G 1 期持续约 9 个小时,S 期持续 10 个小时,G 2 期持续大约四个半小时,M 期持续大约半小时。 在果蝇的早期胚胎中,细胞周期在大约八分钟内完成。 细胞周期中事件的时间由细胞内部和外部机制控制。
外部事件对细胞周期的调节
当细胞即将开始复制过程时,细胞分裂的启动和抑制都是由细胞外部事件触发的。 事件可能像附近细胞的死亡一样简单,也可以像促进生长的激素(例如人类生长激素(HGH)的释放一样广泛。 缺乏生长激素会抑制细胞分裂,导致侏儒症,而过多的生长激素会导致巨人症。 细胞拥挤也会抑制细胞分裂。 另一个可能引发细胞分裂的因素是细胞的大小;随着细胞的生长,由于其表面体积比降低,它变得效率低下。 这个问题的解决方案是分裂。
无论消息的来源如何,小区都会接收信号,小区内的一系列事件允许它进入相间阶段。 从这个起始点开始,必须满足每个细胞周期阶段所需的每个参数,否则循环将无法进行。
内部检查站的监管
所产生的子细胞必须与亲本细胞完全重复。 染色体复制或分布中的错误会导致突变,这些突变可能会传递给由异常细胞产生的每个新细胞。 为了防止受损细胞继续分裂,内部控制机制在三个主要细胞周期检查点运行。 检查点是真核细胞周期中的几个点之一,在这个点上,细胞进入周期的下一个阶段的进展可以停止,直到条件有利为止。 这些检查点出现在 G 1 末尾附近、G 2 /M 过渡时和中期阶段(图\(\PageIndex{1}\))。
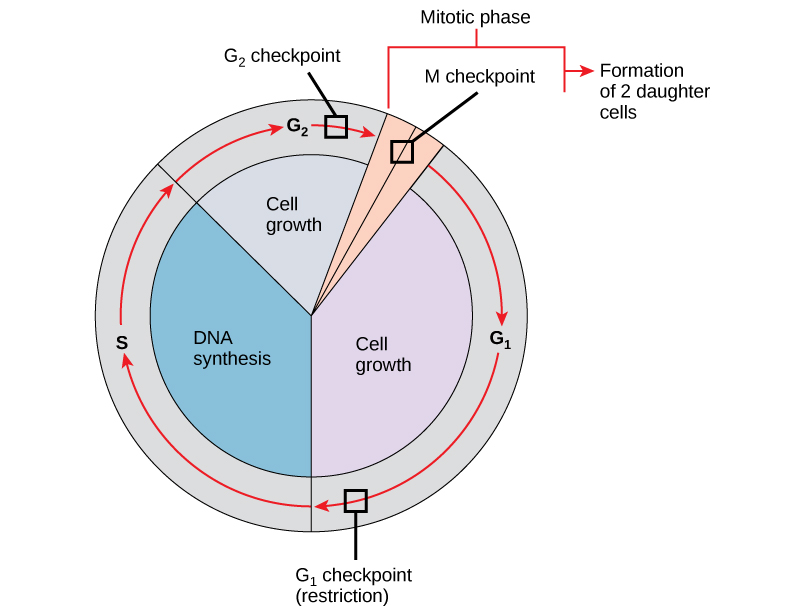
G 1 检查点
G 1 检查点决定是否所有条件都有利于细胞分裂的进行。 G 1 检查点,也称为限制点(酵母中),是细胞不可逆转地投入细胞分裂过程的点。 外部影响,例如生长因子,在使细胞通过 G 1 检查点方面起着重要作用。 除了充足的储备和细胞大小外,还在 G 1 检查点检查基因组 DNA 损伤。 不符合所有要求的电池将不允许进入S阶段。 细胞可以停止循环并尝试纠正有问题的情况,或者在条件改善时,细胞可以进入G 0 并等待进一步的信号。
G 2 检查点
如果不满足某些条件,G 2 检查点会禁止进入有丝分裂阶段。 与G 1 检查点一样,对细胞大小和蛋白质储备进行了评估。 但是,G 2 检查点最重要的作用是确保所有染色体均已复制,复制的 DNA 不会受损。 如果检查点机制检测到 DNA 存在问题,则细胞周期就会停止,细胞会尝试完成 DNA 复制或修复受损的 DNA。
M 检查点
M 检查点发生在 maryokinesis 的中期阶段快要结束时。 M 检查点也被称为主轴检查点,因为它决定了所有姊妹染色体是否正确附着在主轴微管上。 由于在后期阶段分离姊妹染色体是不可逆转的步骤,因此在每对姊妹染色体的动力学牢固地固定在来自细胞两极的至少两根纺锤纤维上之前,循环才会继续。
细胞周期的调节分子
除了内部控制的检查点外,还有两组调节细胞周期的细胞内分子。 这些调节分子要么促进细胞进入下一阶段(正调节),要么停止周期(负调节)。 调节分子可以单独起作用,也可以影响其他调节蛋白的活性或产生。 因此,单个调节器的失效可能对细胞周期几乎没有影响,尤其是在多个机制控制同一个事件的情况下。 相反,调节剂缺陷或失灵的影响可能范围很广,如果多个过程受到影响,则可能对细胞致命。
对细胞周期的积极调节
两组蛋白质,称为细胞周期蛋白和细胞周期蛋白依赖性激酶(Cdk),是细胞通过各种检查点进展的原因。 四种细胞周期蛋白的水平在整个细胞周期中以可预测的模式波动(图\(\PageIndex{2}\)). Increases in the concentration of cyclin proteins are triggered by both external and internal signals. After the cell moves to the next stage of the cell cycle, the cyclins that were active in the previous stage are degraded.
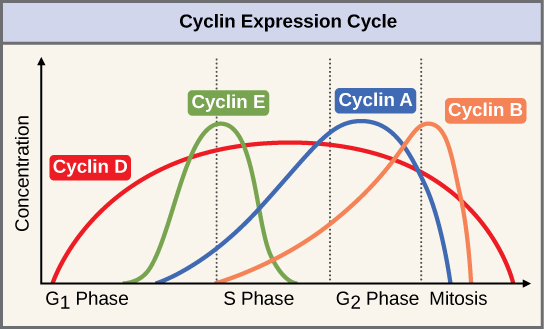
Cyclins regulate the cell cycle only when they are tightly bound to Cdks. To be fully active, the Cdk/cyclin complex must also be phosphorylated in specific locations. Like all kinases, Cdks are enzymes (kinases) that phosphorylate other proteins. Phosphorylation activates the protein by changing its shape. The proteins phosphorylated by Cdks are involved in advancing the cell to the next phase. (Figure \(\PageIndex{3}\)). The levels of Cdk proteins are relatively stable throughout the cell cycle; however, the concentrations of cyclin fluctuate and determine when Cdk/cyclin complexes form. The different cyclins and Cdks bind at specific points in the cell cycle and thus regulate different checkpoints.
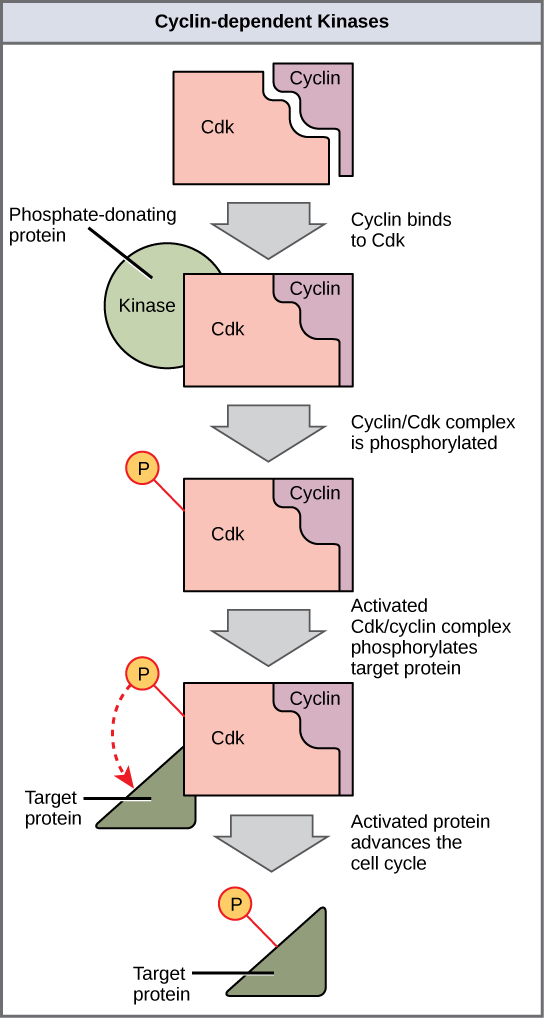
Since the cyclic fluctuations of cyclin levels are based on the timing of the cell cycle and not on specific events, regulation of the cell cycle usually occurs by either the Cdk molecules alone or the Cdk/cyclin complexes. Without a specific concentration of fully activated cyclin/Cdk complexes, the cell cycle cannot proceed through the checkpoints.
Although the cyclins are the main regulatory molecules that determine the forward momentum of the cell cycle, there are several other mechanisms that fine-tune the progress of the cycle with negative, rather than positive, effects. These mechanisms essentially block the progression of the cell cycle until problematic conditions are resolved. Molecules that prevent the full activation of Cdks are called Cdk inhibitors. Many of these inhibitor molecules directly or indirectly monitor a particular cell cycle event. The block placed on Cdks by inhibitor molecules will not be removed until the specific event that the inhibitor monitors is completed.
Negative Regulation of the Cell Cycle
The second group of cell cycle regulatory molecules are negative regulators. Negative regulators halt the cell cycle. Remember that in positive regulation, active molecules cause the cycle to progress.
The best understood negative regulatory molecules are retinoblastoma protein (Rb), p53, and p21. Retinoblastoma proteins are a group of tumor-suppressor proteins common in many cells. The 53 and 21 designations refer to the functional molecular masses of the proteins (p) in kilodaltons. Much of what is known about cell cycle regulation comes from research conducted with cells that have lost regulatory control. All three of these regulatory proteins were discovered to be damaged or non-functional in cells that had begun to replicate uncontrollably (became cancerous). In each case, the main cause of the unchecked progress through the cell cycle was a faulty copy of the regulatory protein.
Rb, p53, and p21 act primarily at the G1 checkpoint. p53 is a multi-functional protein that has a major impact on the commitment of a cell to division because it acts when there is damaged DNA in cells that are undergoing the preparatory processes during G1. If damaged DNA is detected, p53 halts the cell cycle and recruits enzymes to repair the DNA. If the DNA cannot be repaired, p53 can trigger apoptosis, or cell suicide, to prevent the duplication of damaged chromosomes. As p53 levels rise, the production of p21 is triggered. p21 enforces the halt in the cycle dictated by p53 by binding to and inhibiting the activity of the Cdk/cyclin complexes. As a cell is exposed to more stress, higher levels of p53 and p21 accumulate, making it less likely that the cell will move into the S phase.
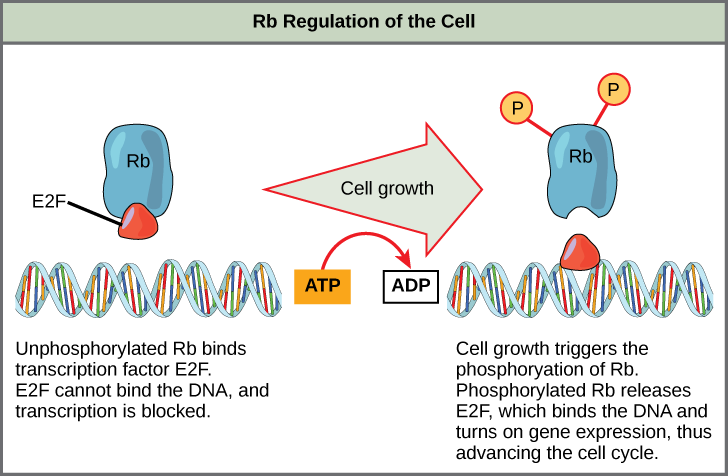
Rb exerts its regulatory influence on other positive regulator proteins. Chiefly, Rb monitors cell size. In the active, dephosphorylated state, Rb binds to proteins called transcription factors, most commonly, E2F (Figure \(\PageIndex{4}\)). Transcription factors “turn on” specific genes, allowing the production of proteins encoded by that gene. When Rb is bound to E2F, production of proteins necessary for the G1/S transition is blocked. As the cell increases in size, Rb is slowly phosphorylated until it becomes inactivated. Rb releases E2F, which can now turn on the gene that produces the transition protein, and this particular block is removed. For the cell to move past each of the checkpoints, all positive regulators must be “turned on,” and all negative regulators must be “turned off.”
Exercise \(\PageIndex{1}\)
Rb and other proteins that negatively regulate the cell cycle are sometimes called tumor suppressors. Why do you think the name tumor suppressor might be appropriate for these proteins?
- Answer
-
Rb and other negative regulatory proteins control cell division and therefore prevent the formation of tumors. Mutations that prevent these proteins from carrying out their function can result in cancer.
Summary
Each step of the cell cycle is monitored by internal controls called checkpoints. There are three major checkpoints in the cell cycle: one near the end of G1, a second at the G2/M transition, and the third during metaphase. Positive regulator molecules allow the cell cycle to advance to the next stage. Negative regulator molecules monitor cellular conditions and can halt the cycle until specific requirements are met.
Glossary
- cell cycle checkpoint
- mechanism that monitors the preparedness of a eukaryotic cell to advance through the various cell cycle stages
- cyclin
- one of a group of proteins that act in conjunction with cyclin-dependent kinases to help regulate the cell cycle by phosphorylating key proteins; the concentrations of cyclins fluctuate throughout the cell cycle
- cyclin-dependent kinase
- one of a group of protein kinases that helps to regulate the cell cycle when bound to cyclin; it functions to phosphorylate other proteins that are either activated or inactivated by phosphorylation
- p21
- cell cycle regulatory protein that inhibits the cell cycle; its levels are controlled by p53
- p53
- cell cycle regulatory protein that regulates cell growth and monitors DNA damage; it halts the progression of the cell cycle in cases of DNA damage and may induce apoptosis
- retinoblastoma protein (Rb)
- regulatory molecule that exhibits negative effects on the cell cycle by interacting with a transcription factor (E2F)



