10.3: Udhibiti wa Mzunguko wa Kiini
- Page ID
- 176044
Ujuzi wa Kuendeleza
- Kuelewa jinsi mzunguko wa seli unadhibitiwa na taratibu za ndani na nje kwa seli
- Eleza jinsi vitu vitatu vya udhibiti wa ndani vinatokea mwishoni mwa G 1, katika mpito wa G 2/M, na wakati wa metapase
- Eleza molekuli zinazodhibiti mzunguko wa seli kupitia kanuni nzuri na hasi
Urefu wa mzunguko wa seli ni tofauti sana, hata ndani ya seli za kiumbe kimoja. Kwa binadamu, mzunguko wa mauzo ya seli ni kati ya masaa machache katika maendeleo ya awali ya embryonic, kwa wastani wa siku mbili hadi tano kwa seli epithelial, na kwa maisha yote ya binadamu alitumia katika G 0 na seli maalumu, kama vile neurons gamba au seli za misuli ya moyo. Pia kuna tofauti wakati ambapo kiini hutumia katika kila awamu ya mzunguko wa seli. Wakati seli za mamalia zinazogawanya haraka zinapandwa katika utamaduni (nje ya mwili chini ya hali bora ya kukua), urefu wa mzunguko ni takriban masaa 24. Katika kugawa haraka seli za binadamu na mzunguko wa seli ya saa 24, awamu ya G 1 huchukua takriban masaa tisa, awamu ya S huchukua masaa 10, awamu ya G 2 huchukua muda wa saa nne na nusu, na awamu ya M huchukua takriban nusu saa. Katika majani mapema ya nzizi za matunda, mzunguko wa seli umekamilika kwa muda wa dakika nane. Muda wa matukio katika mzunguko wa seli hudhibitiwa na taratibu ambazo ni za ndani na nje kwa seli.
Udhibiti wa Mzunguko wa Kiini na Matukio ya Nje
Wote uanzishwaji na kuzuia mgawanyiko wa seli husababishwa na matukio ya nje ya kiini wakati inakaribia kuanza mchakato wa kuiga. Tukio inaweza kuwa rahisi kama kifo cha kiini jirani au kama yanayojitokeza kama kutolewa kwa homoni ukuaji wa kukuza, kama vile binadamu uchumi homoni (HGH). Ukosefu wa HGH unaweza kuzuia mgawanyiko wa seli, kusababisha dwarfism, ambapo sana HGH inaweza kusababisha gigantism. Kuongezeka kwa seli pia kunaweza kuzuia mgawanyiko wa seli. Sababu nyingine ambayo inaweza kuanzisha mgawanyiko wa seli ni ukubwa wa seli; kama seli inakua, inakuwa haina ufanisi kutokana na kupungua kwa uwiano wake wa uso kwa kiasi. Suluhisho la tatizo hili ni kugawanya.
Chochote chanzo cha ujumbe, kiini hupokea ishara, na mfululizo wa matukio ndani ya seli huruhusu kuendelea kwenye interphase. Kuendelea mbele kutoka hatua hii ya uanzishaji, kila parameter inahitajika wakati wa kila awamu ya mzunguko wa seli lazima ifikiwe au mzunguko hauwezi kuendelea.
Udhibiti katika Vituo vya ukaguzi
Ni muhimu kwamba seli za binti zinazozalishwa kuwa duplicates halisi ya kiini cha mzazi. Makosa katika kurudia au usambazaji wa chromosomes husababisha mabadiliko ambayo yanaweza kupitishwa mbele kwa kila seli mpya zinazozalishwa kutoka seli isiyo ya kawaida. Ili kuzuia kiini kilichoathiriwa kuendelea kugawanya, kuna mifumo ya udhibiti wa ndani ambayo inafanya kazi katika vituo vya ukaguzi vitatu vya mzunguko wa kiini. Checkpoint ni moja ya pointi kadhaa katika mzunguko wa seli ya eukaryotic ambapo maendeleo ya seli hadi hatua inayofuata katika mzunguko inaweza kusimamishwa mpaka hali ni nzuri. Vituo vya ukaguzi hivi hutokea karibu na mwisho wa G 1, katika mabadiliko ya G 2 /M, na wakati wa metapase (Kielelezo\(\PageIndex{1}\)).
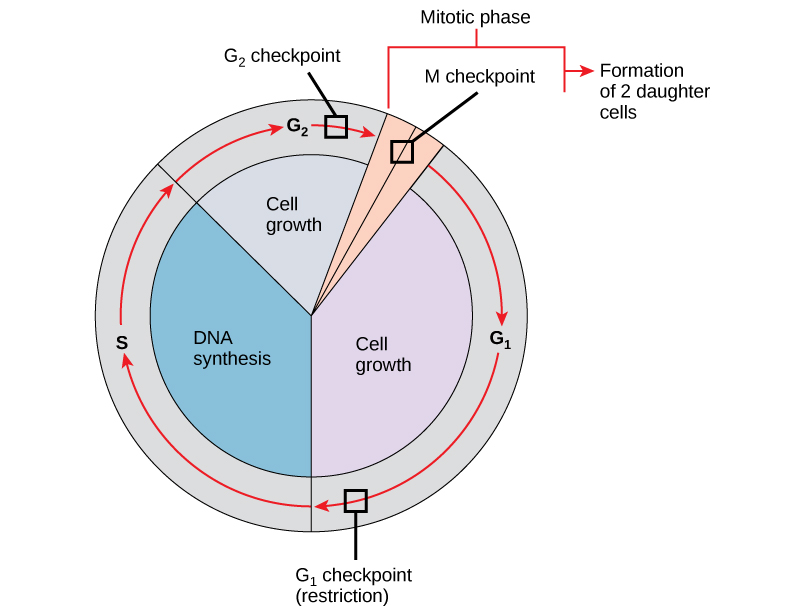
Checkpoint G 1
Checkpoint G 1 huamua kama hali zote zinafaa kwa mgawanyiko wa seli kuendelea. Checkpoint G 1, pia huitwa hatua ya kizuizi (katika chachu), ni hatua ambayo kiini kinatenda kwa mchakato wa mgawanyiko wa seli. Mvuto wa nje, kama vile sababu za ukuaji, una jukumu kubwa katika kubeba kiini kilichopita kituo cha G 1. Mbali na hifadhi ya kutosha na ukubwa wa seli, kuna hundi ya uharibifu wa DNA ya genomic kwenye checkpoint ya G 1. Kiini ambacho hakikidhi mahitaji yote haitaruhusiwa kuendelea katika awamu ya S. Kiini kinaweza kuzuia mzunguko na kujaribu kurekebisha hali ya shida, au kiini kinaweza kuendeleza ndani ya G 0 na kusubiri ishara zaidi wakati hali inaboresha.
Checkpoint G 2
Viwango vya ukaguzi vya G 2 vinaingia kwenye awamu ya mitotic ikiwa hali fulani hazipatikani. Kama katika checkpoint G 1, ukubwa wa seli na hifadhi ya protini ni tathmini. Hata hivyo, jukumu muhimu zaidi la checkpoint G 2 ni kuhakikisha kwamba chromosomes zote zimeandaliwa na kwamba DNA iliyoigwa haiharibiki. Ikiwa utaratibu wa ukaguzi hugundua matatizo na DNA, mzunguko wa seli umesitishwa, na kiini kinajaribu kukamilisha replication ya DNA au kutengeneza DNA iliyoharibiwa.
M Checkpoint
Checkpoint M hutokea karibu na mwisho wa hatua ya metapase ya karyokinesis. Mtazamo wa M pia unajulikana kama checkpoint ya spindle, kwa sababu huamua kama chromatids zote za dada zimeunganishwa kwa usahihi kwenye microtubules ya spindle. Kwa sababu mgawanyo wa chromatids dada wakati wa anaphase ni hatua Malena, mzunguko hautaendelea mpaka kinetochores ya kila jozi ya chromatids dada imara nanga kwa nyuzi mbili spindle inayotokana na miti kinyume ya seli.
Unganisha na Kujifunza

Tazama kile kinachotokea kwenye vituo vya ukaguzi vya G 1, G 2, na M kwa kutembelea tovuti hii ili kuona uhuishaji wa mzunguko wa seli.
Mdhibiti Molekuli ya Mzunguko wa Kiini
Mbali na vituo vya ukaguzi vilivyodhibitiwa ndani, kuna makundi mawili ya molekuli za intracellular zinazodhibiti mzunguko wa seli. Molekuli hizi za udhibiti ama kukuza maendeleo ya seli kwa awamu inayofuata (kanuni chanya) au kusimamisha mzunguko (kanuni hasi). Molekuli ya mdhibiti inaweza kutenda moja kwa moja, au wanaweza kuathiri shughuli au uzalishaji wa protini nyingine za udhibiti. Kwa hiyo, kushindwa kwa mdhibiti mmoja kunaweza kuwa na athari yoyote kwenye mzunguko wa seli, hasa ikiwa utaratibu zaidi ya moja hudhibiti tukio moja. Kinyume chake, athari za mdhibiti wa upungufu au usio na kazi inaweza kuwa pana na uwezekano wa kusababisha kifo kwa seli ikiwa michakato nyingi huathiriwa.
Udhibiti mzuri wa Mzunguko wa Kiini
Makundi mawili ya protini, yanayoitwa cyclins na kinases tegemezi ya baiskeli (Cdks), huwajibika kwa maendeleo ya seli kupitia vituo mbalimbali vya ukaguzi. Viwango vya protini nne za cyclin hubadilishana katika mzunguko wa seli katika muundo wa kutabirika (Kielelezo\(\PageIndex{2}\)). Increases in the concentration of cyclin proteins are triggered by both external and internal signals. After the cell moves to the next stage of the cell cycle, the cyclins that were active in the previous stage are degraded.
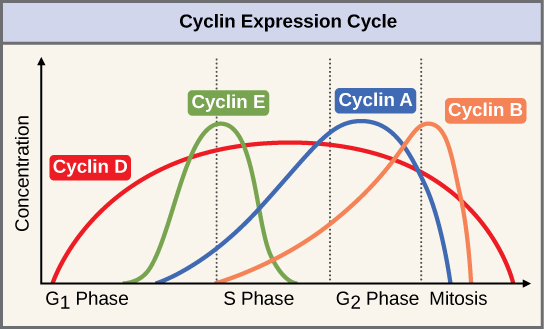
Cyclins regulate the cell cycle only when they are tightly bound to Cdks. To be fully active, the Cdk/cyclin complex must also be phosphorylated in specific locations. Like all kinases, Cdks are enzymes (kinases) that phosphorylate other proteins. Phosphorylation activates the protein by changing its shape. The proteins phosphorylated by Cdks are involved in advancing the cell to the next phase. (Figure \(\PageIndex{3}\)). The levels of Cdk proteins are relatively stable throughout the cell cycle; however, the concentrations of cyclin fluctuate and determine when Cdk/cyclin complexes form. The different cyclins and Cdks bind at specific points in the cell cycle and thus regulate different checkpoints.
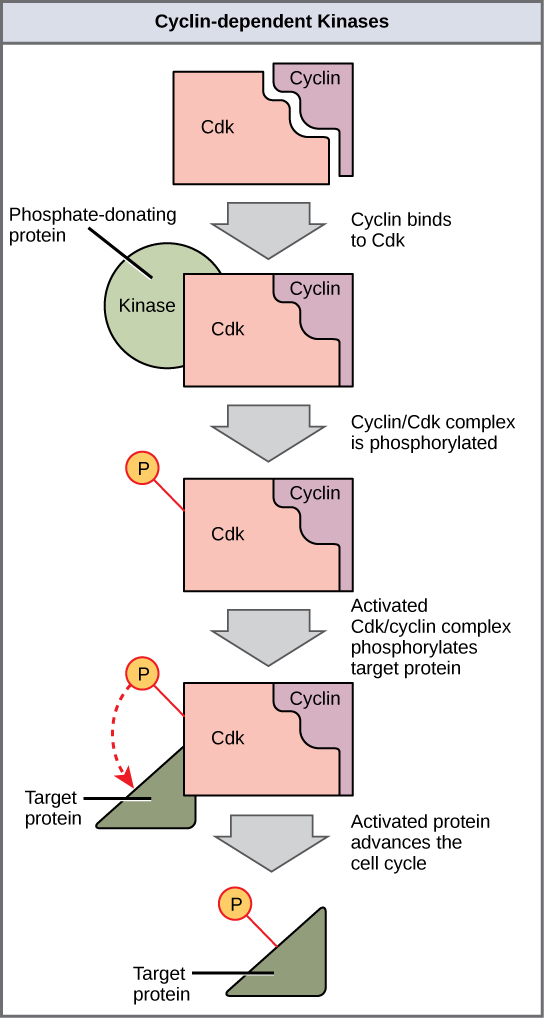
Since the cyclic fluctuations of cyclin levels are based on the timing of the cell cycle and not on specific events, regulation of the cell cycle usually occurs by either the Cdk molecules alone or the Cdk/cyclin complexes. Without a specific concentration of fully activated cyclin/Cdk complexes, the cell cycle cannot proceed through the checkpoints.
Although the cyclins are the main regulatory molecules that determine the forward momentum of the cell cycle, there are several other mechanisms that fine-tune the progress of the cycle with negative, rather than positive, effects. These mechanisms essentially block the progression of the cell cycle until problematic conditions are resolved. Molecules that prevent the full activation of Cdks are called Cdk inhibitors. Many of these inhibitor molecules directly or indirectly monitor a particular cell cycle event. The block placed on Cdks by inhibitor molecules will not be removed until the specific event that the inhibitor monitors is completed.
Negative Regulation of the Cell Cycle
The second group of cell cycle regulatory molecules are negative regulators. Negative regulators halt the cell cycle. Remember that in positive regulation, active molecules cause the cycle to progress.
The best understood negative regulatory molecules are retinoblastoma protein (Rb), p53, and p21. Retinoblastoma proteins are a group of tumor-suppressor proteins common in many cells. The 53 and 21 designations refer to the functional molecular masses of the proteins (p) in kilodaltons. Much of what is known about cell cycle regulation comes from research conducted with cells that have lost regulatory control. All three of these regulatory proteins were discovered to be damaged or non-functional in cells that had begun to replicate uncontrollably (became cancerous). In each case, the main cause of the unchecked progress through the cell cycle was a faulty copy of the regulatory protein.
Rb, p53, and p21 act primarily at the G1 checkpoint. p53 is a multi-functional protein that has a major impact on the commitment of a cell to division because it acts when there is damaged DNA in cells that are undergoing the preparatory processes during G1. If damaged DNA is detected, p53 halts the cell cycle and recruits enzymes to repair the DNA. If the DNA cannot be repaired, p53 can trigger apoptosis, or cell suicide, to prevent the duplication of damaged chromosomes. As p53 levels rise, the production of p21 is triggered. p21 enforces the halt in the cycle dictated by p53 by binding to and inhibiting the activity of the Cdk/cyclin complexes. As a cell is exposed to more stress, higher levels of p53 and p21 accumulate, making it less likely that the cell will move into the S phase.
Rb exerts its regulatory influence on other positive regulator proteins. Chiefly, Rb monitors cell size. In the active, dephosphorylated state, Rb binds to proteins called transcription factors, most commonly, E2F (Figure \(\PageIndex{4}\)). Transcription factors “turn on” specific genes, allowing the production of proteins encoded by that gene. When Rb is bound to E2F, production of proteins necessary for the G1/S transition is blocked. As the cell increases in size, Rb is slowly phosphorylated until it becomes inactivated. Rb releases E2F, which can now turn on the gene that produces the transition protein, and this particular block is removed. For the cell to move past each of the checkpoints, all positive regulators must be “turned on,” and all negative regulators must be “turned off.”
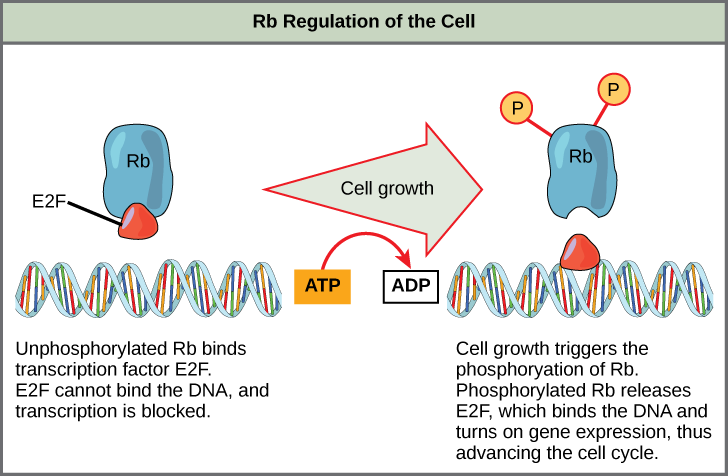
Exercise \(\PageIndex{1}\)
Rb and other proteins that negatively regulate the cell cycle are sometimes called tumor suppressors. Why do you think the name tumor suppressor might be appropriate for these proteins?
- Answer
-
Rb and other negative regulatory proteins control cell division and therefore prevent the formation of tumors. Mutations that prevent these proteins from carrying out their function can result in cancer.
Summary
Each step of the cell cycle is monitored by internal controls called checkpoints. There are three major checkpoints in the cell cycle: one near the end of G1, a second at the G2/M transition, and the third during metaphase. Positive regulator molecules allow the cell cycle to advance to the next stage. Negative regulator molecules monitor cellular conditions and can halt the cycle until specific requirements are met.
Glossary
- cell cycle checkpoint
- mechanism that monitors the preparedness of a eukaryotic cell to advance through the various cell cycle stages
- cyclin
- one of a group of proteins that act in conjunction with cyclin-dependent kinases to help regulate the cell cycle by phosphorylating key proteins; the concentrations of cyclins fluctuate throughout the cell cycle
- cyclin-dependent kinase
- one of a group of protein kinases that helps to regulate the cell cycle when bound to cyclin; it functions to phosphorylate other proteins that are either activated or inactivated by phosphorylation
- p21
- cell cycle regulatory protein that inhibits the cell cycle; its levels are controlled by p53
- p53
- cell cycle regulatory protein that regulates cell growth and monitors DNA damage; it halts the progression of the cell cycle in cases of DNA damage and may induce apoptosis
- retinoblastoma protein (Rb)
- regulatory molecule that exhibits negative effects on the cell cycle by interacting with a transcription factor (E2F)


