29.1: Quantization ya Nishati
- Page ID
- 183549
Malengo ya kujifunza
Mwishoni mwa sehemu hii, utaweza:
- Eleza mchango wa Max Planck katika maendeleo ya mechanics quantum.
- Eleza kwa nini spectra ya atomiki inaonyesha quantization.
Mchango wa Planck
Nishati ni quantized katika baadhi ya mifumo, maana kwamba mfumo unaweza kuwa na nguvu fulani tu na si kuendelea kwa nguvu, tofauti na kesi classical. Hii itakuwa kama kuwa na kasi fulani tu ambayo gari inaweza kusafiri kwa sababu nishati yake ya kinetic inaweza kuwa na maadili fulani tu. Pia tunaona kwamba aina fulani za uhamisho wa nishati hufanyika na uvimbe wa nishati. Wakati wengi wetu ni ukoo na quantization ya suala katika uvimbe kuitwa atomi, molekuli, na kadhalika, sisi ni chini ya ufahamu kwamba nishati, pia, inaweza kuwa quantized. Baadhi ya dalili za mwanzo juu ya umuhimu wa mechanics quantum juu ya fizikia classical alikuja kutoka quantization ya nishati.
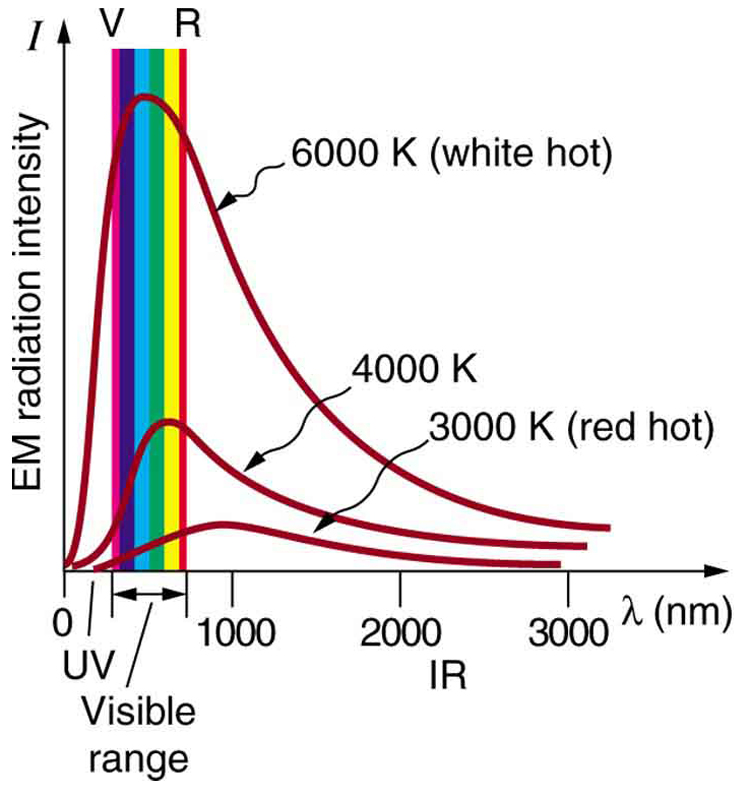
Where is the quantization of energy observed? Let us begin by considering the emission and absorption of electromagnetic (EM) radiation. The EM spectrum radiated by a hot solid is linked directly to the solid’s temperature (Figure \(\PageIndex{1}\)) An ideal radiator is one that has an emissivity of 1 at all wavelengths and, thus, is jet black. Ideal radiators are therefore called blackbodies, and their EM radiation is called blackbody radiation. It was discussed that the total intensity of the radiation varies as \(T^4\), the fourth power of the absolute temperature of the body, and that the peak of the spectrum shifts to shorter wavelengths at higher temperatures. All of this seems quite continuous, but it was the curve of the spectrum of intensity versus wavelength that gave a clue that the energies of the atoms in the solid are quantized. In fact, providing a theoretical explanation for the experimentally measured shape of the spectrum was a mystery at the turn of the century. When this “ultraviolet catastrophe” was eventually solved, the answers led to new technologies such as computers and the sophisticated imaging techniques described in earlier chapters. Once again, physics as an enabling science changed the way we live.
The German physicist Max Planck (1858–1947) used the idea that atoms and molecules in a body act like oscillators to absorb and emit radiation. The energies of the oscillating atoms and molecules had to be quantized to correctly describe the shape of the blackbody spectrum. Planck deduced that the energy of an oscillator having a frequency \(f\) is given by
\[E = \left(n + \dfrac{1}{2} \right) hf.\]
Here \(n\) is any nonnegative integer (0, 1, 2, 3, …). The symbol \(h\) stands for Planck’s constant, given by
\[h = 6.626 \times 10^{-34} \, J \cdot s.\] The equation \(E = (n + \frac{1}{2}) hf\) means that an oscillator having a frequency \(f\) (emitting and absorbing EM radiation of frequency \(f\)) can have its energy increase or decrease only in discrete steps of size
\[\Delta E = hf.\]
It might be helpful to mention some macroscopic analogies of this quantization of energy phenomena. This is like a pendulum that has a characteristic oscillation frequency but can swing with only certain amplitudes. Quantization of energy also resembles a standing wave on a string that allows only particular harmonics described by integers. It is also similar to going up and down a hill using discrete stair steps rather than being able to move up and down a continuous slope. Your potential energy takes on discrete values as you move from step to step.
Using the quantization of oscillators, Planck was able to correctly describe the experimentally known shape of the blackbody spectrum. This was the first indication that energy is sometimes quantized on a small scale and earned him the Nobel Prize in Physics in 1918. Although Planck’s theory comes from observations of a macroscopic object, its analysis is based on atoms and molecules. It was such a revolutionary departure from classical physics that Planck himself was reluctant to accept his own idea that energy states are not continuous. The general acceptance of Planck’s energy quantization was greatly enhanced by Einstein’s explanation of the photoelectric effect (discussed in the next section), which took energy quantization a step further. Planck was fully involved in the development of both early quantum mechanics and relativity. He quickly embraced Einstein’s special relativity, published in 1905, and in 1906 Planck was the first to suggest the correct formula for relativistic momentum, \(p = \gamma mu\).

Kumbuka kuwa mara kwa mara ya Planck\(h\) ni idadi ndogo sana. Hivyo kwa mzunguko wa infrared\(10^{14}\) wa kutolewa na blackbody, kwa mfano, tofauti kati ya viwango vya nishati ni tu\(\Delta E = hf = (6.63 \times 10^{-34} \, J \cdot s)(10^{14} \, Hz) = 6.63 \times 10^{-20} \, J\), au kuhusu 0.4 eV. Hii 0.4 eV ya nishati ni muhimu ikilinganishwa na nguvu za kawaida za atomiki, ambazo ziko juu ya utaratibu wa volt ya elektroni, au nguvu za joto, ambazo ni kawaida sehemu ndogo za volt ya elektroni. Lakini kwa kiwango kikubwa au classical, nguvu ni kawaida juu ya utaratibu wa joules. Hata kama nguvu za macroscopic zinapimwa, hatua za quantum ni ndogo sana kutambuliwa. Hii ni mfano wa kanuni ya mawasiliano. Kwa kitu kikubwa, mechanics quantum hutoa matokeo kutofautishwa na yale ya fizikia classical.
Atomiki Spectra
Sasa hebu tugeuze mawazo yetu kwa chafu na ngozi ya mionzi ya EM na gesi. Jua ni mfano wa kawaida wa mwili ulio na gesi zinazotoa wigo wa EM unaojumuisha nuru inayoonekana. Pia tunaona mifano katika ishara za neon na moto wa mishumaa. Uchunguzi wa uzalishaji wa gesi za moto ulianza zaidi ya karne mbili zilizopita, na hivi karibuni ilitambuliwa kuwa spectra hizi za uchafu zilikuwa na kiasi kikubwa cha habari. Aina ya gesi na joto lake, kwa mfano, inaweza kuamua. Sasa tunajua kwamba uzalishaji huu wa EM unatokana na elektroni zinazopita kati ya viwango vya nishati katika atomi binafsi na molekuli; hivyo, huitwa spectra atomiki. Spectra ya atomiki inabakia chombo muhimu cha uchambuzi leo. Kielelezo\(\PageIndex{3}\) kinaonyesha mfano wa wigo wa chafu uliopatikana kwa kupitisha kutokwa kwa umeme kupitia nyenzo. Moja ya sifa muhimu zaidi za spectra hizi ni kwamba wao ni wazi. Kwa hili tunamaanisha kwamba wavelengths fulani tu, na hivyo masafa, hutolewa. Hii inaitwa wigo wa mstari. Kama frequency na nishati ni kuhusishwa kama\(\Delta E = hf\), nguvu za elektroni katika atomi emitting na molekuli ni quantized. Hii inajadiliwa kwa undani zaidi baadaye katika sura hii.

It was a major puzzle that atomic spectra are quantized. Some of the best minds of 19th-century science failed to explain why this might be. Not until the second decade of the 20th century did an answer based on quantum mechanics begin to emerge. Again a macroscopic or classical body of gas was involved in the studies, but the effect, as we shall see, is due to individual atoms and molecules.
PHET EXPLORATIONS: MODELS OF THE HYDROGEN ATOM
How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.
![]()
Summary
- The first indication that energy is sometimes quantized came from blackbody radiation, which is the emission of EM radiation by an object with an emissivity of 1.
- Planck recognized that the energy levels of the emitting atoms and molecules were quantized, with only the allowed values of \(E = (n + \frac{1}{2})hf\), where \(n\) is any non-negative integer (0, 1, 2, 3, …).
- \(h\) is Planck’s constant, whose value is \(h = 6.626 \times 10^{-34} \, J \cdot s\).
- Thus, the oscillatory absorption and emission energies of atoms and molecules in a blackbody could increase or decrease only in steps of size \(\Delta E = hf\) where \(f\) is the frequency of the oscillatory nature of the absorption and emission of EM radiation.
- Another indication of energy levels being quantized in atoms and molecules comes from the lines in atomic spectra, which are the EM emissions of individual atoms and molecules.
Glossary
- blackbody
- an ideal radiator, which can radiate equally well at all wavelengths
- blackbody radiation
- the electromagnetic radiation from a blackbody
- Planck’s constant
- \(6.626 \times 10^{-34} \, J \cdot s\)
- atomic spectra
- the electromagnetic emission from atoms and molecules


