12.E: Kinetics (Mazoezi)
- Page ID
- 176470
12.1: Viwango vya mmenyuko wa Kemikali
Q12.1.1
Ni tofauti gani kati ya kiwango cha wastani, kiwango cha awali, na kiwango cha instantaneous?
- Suluhisho
-
Kwanza, kiwango cha majibu ya jumla kinapaswa kuelezwa kujua nini tofauti yoyote ya kiwango ni. Kiwango cha mmenyuko kinafafanuliwa kama kipimo cha mabadiliko katika mkusanyiko wa reactants au bidhaa kwa wakati wa kitengo. Kiwango cha mmenyuko wa kemikali sio mabadiliko ya mara kwa mara na badala yake, na inaweza kuathiriwa na joto. Kiwango cha mmenyuko kinaweza kuelezwa kama kutoweka kwa reactant yoyote au kuonekana kwa bidhaa yoyote. Hivyo, kiwango cha wastani ni wastani wa kiwango cha mmenyuko katika kipindi fulani cha muda katika mmenyuko, kiwango cha instantaneous ni kiwango cha mmenyuko kwa wakati fulani wakati wa majibu, na kiwango cha awali ni kiwango cha instantaneous mwanzoni mwa majibu (wakati bidhaa huanza kuunda).
Kiwango cha instantaneous cha mmenyuko kinaweza kuashiria kama\[ \lim_{\Delta t \rightarrow 0} \dfrac{\Delta [concentration]}{\Delta t} \nonumber \]
Q12.1.2
Ozone hutengana na oksijeni kulingana na equation\(\ce{2O3}(g)⟶\ce{3O2}(g)\). Andika equation inayohusiana na maneno ya kiwango cha mmenyuko huu kwa suala la kutoweka kwa O 3 na kuundwa kwa oksijeni.
- Suluhisho
-
Kwa majibu ya jumla, aA —> bB, kiwango cha mmenyuko kinaweza kuelezwa kwa suala la kutoweka kwa A au kuonekana kwa B kwa kipindi fulani cha wakati kama ifuatavyo.
\[- \dfrac{1}{a}\dfrac{\Delta [A]}{\Delta t} = - \dfrac{1}{b}\dfrac{\Delta [B]}{\Delta t} = \dfrac{1}{c}\dfrac{\Delta [C]}{\Delta t} = \dfrac{1}{d}\dfrac{\Delta [D]}{\Delta t}\]
Tunataka kiwango cha mmenyuko kuwa chanya, lakini mabadiliko katika mkusanyiko wa mmenyuko, A, itakuwa hasi kwa sababu inatumiwa hadi kugeuzwa kuwa bidhaa, Kwa hiyo, wakati wa kuonyesha kiwango cha majibu kwa suala la mabadiliko katika mkusanyiko wa A, ni muhimu kuongeza hasi ishara mbele ili kuhakikisha kiwango cha jumla chanya.
Hatimaye, kiwango lazima kiwe kawaida kulingana na stoichiometry ya mmenyuko. Katika utengano wa ozoni kwa oksijeni, moles mbili za ozoni huunda moles tatu za gesi ya oksijeni. Hii ina maana kwamba ongezeko la gesi ya oksijeni itakuwa mara 1.5 kubwa kama kupungua kwa ozoni. Kwa sababu kiwango cha mmenyuko kinapaswa kuwa na uwezo wa kuelezea aina zote mbili, tunagawanya mabadiliko katika mkusanyiko na mgawo wake wa stoichiometric katika usawa wa majibu ya kukabiliana na suala hili.
Kwa hiyo, kiwango cha mmenyuko wa kuharibika kwa ozoni ndani ya gesi ya oksijeni inaweza kuelezewa kama ifuatavyo:
\[Rate=-\frac{Δ[O3]}{2ΔT}=\frac{Δ[O2]}{3ΔT}\]
- Jibu
-
$Kiwango=-\ frac {Δ [O3]} {2ΔT} =\ frac {Δ [O2]} {3ΔT}\]
Q12.1.3
Katika sekta ya nyuklia, trifluoride ya klorini hutumiwa kuandaa hexafluoride ya uranium, kiwanja kikubwa cha uranium kinachotumiwa katika kutenganishwa kwa isotopu za uranium. Chlorini trifluoride imeandaliwa na mmenyuko\(\ce{Cl2}(g)+\ce{3F2}(g)⟶\ce{2ClF3}(g)\). Andika equation inayohusiana na maneno ya kiwango cha mmenyuko huu kwa suala la kutoweka kwa Cl 2 na F 2 na malezi ya ClF 3.
- Suluhisho
-
Katika tatizo hili tunaulizwa kuandika equation ambayo inahusiana maneno ya kiwango katika suala la kutoweka kwa reactants ya equation na katika suala la malezi ya bidhaa. Kiwango cha mmenyuko hutoa ufahamu wa jinsi kiwango kinachoathirika kama kazi ya mkusanyiko wa vitu katika equation. Viwango mara nyingi huweza kuonyeshwa kwenye grafu za mkusanyiko vs wakati ulioonyeshwa katika mabadiliko (\({\Delta}\)) ya mkusanyiko na wakati na kwa muda mfupi wa kutosha, kiwango cha instantaneous kinaweza kuhesabiwa. Kama tulikuwa na kuchambua majibu yaliyotolewa, grafu ingeonyesha kuwa Cl 2 itapungua, kwamba F 2 itapungua mara 3 kwa haraka, na kisha ClF 3 huongezeka kwa kiwango cha mara mbili. Majibu yanatumiwa na kubadilishwa kuwa bidhaa hivyo hupungua wakati bidhaa zinaongezeka.
Kwa tatizo hili, tunaweza kutumia formula ya jumla ya kiwango kwa masuala maalum ya tatizo ambapo fomu ya jumla ifuatavyo:\[aA+bB⟶cC+dD\nonumber \].
Na kiwango kinaweza kuandikwa kama\(rate=-\frac {1}{a}\frac{{\Delta}[A]}{{\Delta}t}\)\(=-\frac {1}{b}\frac{{\Delta}[B]}{{\Delta}t}\)\(=\frac {1}{c}\frac{{\Delta}[C]}{{\Delta}t}\)\(=\frac {1}{d}\frac{{\Delta}[D]}{{\Delta}t}.\) Hapa ishara hasi hutumiwa kuweka mkataba wa kueleza viwango kama namba chanya.
Katika kesi hii maalum tunatumia stoichiometry kupata viwango maalum vya kutoweka na malezi (nyuma ya kile kilichosemwa katika aya ya kwanza). Hivyo, tatizo tu inahusisha akimaanisha equation na coefficients yake uwiano. Kulingana na equation tunaona kwamba Cl 2 ni reactant na haina mgawo, F 2 ina mgawo wa 3 na pia hutumiwa juu, na kisha ClF 3 ni bidhaa inayoongezeka mara mbili na mgawo wa 2. Kwa hiyo, kiwango cha hapa kinaweza kuandikwa kama:\[rate=-\frac{{\Delta}[Cl_2]}{{\Delta}t}=-\frac {1}{3}\frac{{\Delta}[F_2]}{{\Delta}t}=\frac {1}{2}\frac{{\Delta}[ClF_3]}{{\Delta}t}\nonumber \]
- Jibu
-
\[\ce{rate}=+\dfrac{1}{2}\dfrac{Δ[\ce{CIF3}]}{Δt}=−\dfrac{Δ[\ce{Cl2}]}{Δt}=−\dfrac{1}{3}\dfrac{Δ[\ce{F2}]}{Δt}\nonumber \]
Q12.1.4
Utafiti wa kiwango cha dimerization ya C 4 H 6 alitoa data iliyoonyeshwa katika meza:
\[\ce{2C4H6⟶C8H12}\nonumber \]
| Muda (s) | 0 | 1600 | 3200 | 4800 | 6200 |
|---|---|---|---|---|---|
| [C 4 H 6] (M) | 1.00 × 10 -1 -2 | 5.04 × 10 -3 | 3.37 × 10 -3 | 2.53 × 10 -3 | 2.08 × 10 -3 |
- Kuamua kiwango cha wastani cha dimerization kati ya 0 s na 1600 s, na kati ya 1600 s na 3200 s.
- Tathmini kiwango cha instantaneous cha dimerization saa 3200 s kutoka grafu ya muda dhidi ya [C 4 H 6]. Je, ni vitengo vya kiwango hiki?
- Kuamua kiwango cha wastani cha malezi ya C 8 H 12 saa 1600 s na kiwango cha malezi ya papo hapo saa 3200 s kutoka viwango vilivyopatikana katika sehemu (a) na (b).
- Suluhisho
-
1.) Kiwango cha wastani cha dimerization ni mabadiliko katika mkusanyiko wa reactant kwa wakati wa kitengo. Katika kesi hii itakuwa:
\(rate\)\(of\)\(dimerization=-\frac{\Delta [C_4H_6]}{\Delta t}\)
Kiwango cha dimerization kati ya 0 s na 1600 s:
\(rate\)\(of\)\(dimerization=-\frac{5.04×10^{-3}M-1.00×10^{-2}M}{1600 s-0 s}\)
\(rate\)\(of\)\(dimerization=3.10 × 10^{-6} \frac{M}{s}\)
Kiwango cha dimerization kati ya 1600 s na 3200 s:
\(rate\)\(of\)\(dimerization=-\frac{3.37×10^{-3}M-5.04×10^{-3}M}{3200 s-1600 s}\)
\(rate\)\(of\)\(dimerization=1.04 × 10^{-6} \frac{M}{s}\)
2.) Kiwango cha instantaneous cha dimerization saa 3200 s kinaweza kupatikana kwa wakati wa kuchora dhidi ya [C 4 H 6].
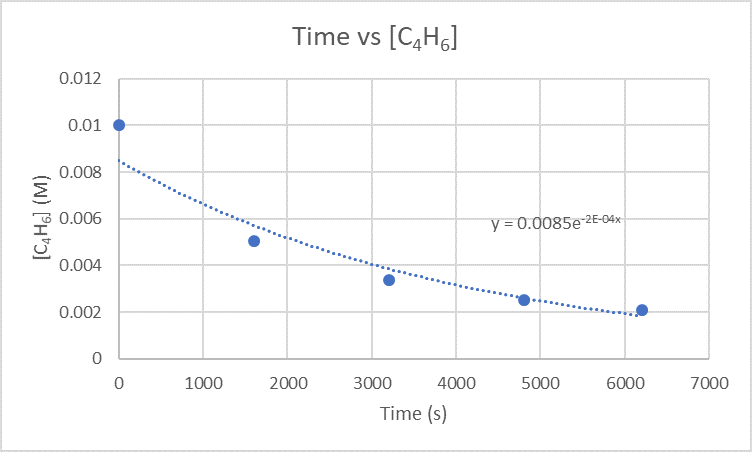
Kwa sababu unataka kupata kiwango cha dimerization saa 3200 s, unahitaji kupata mteremko kati ya 1600 s na 3200 s na pia 3200 s na 4800 s.
Kwa mteremko kati ya 1600 s na 3200 s kutumia pointi (1600 s, 5.04 x 10 -3 M) na (3200 s, 3.37 x 10 -3 M)
\(\frac{3.37×10^{-3}M-5.04×10^{-3}M}{3200 s-1600 s}\)
\(\frac{-0.00167 M}{1600 s}\)
\(-1.04×10^{-6}\frac{M}{s}\)
Kwa mteremko kati ya 3200 s na 4800 s kutumia pointi (3200s, 3.37 x 10 -3 M) na (4800s, 2.53 x 10 -3 M)
\(\frac{2.53×10^{-3}M-3.37×10^{-3}M}{4800 s-3200 s}\)
\(\frac{-8.4×10^{-4} M}{1600 s}\)
\(-5.25×10^{-7}\frac{M}{s}\)
Kuchukua mteremko mbili tu kupatikana na kupata wastani wao kupata kiwango instantaneous ya dimerization.
\(\frac{-1.04×10^{-6}\frac{M}{s}+-5.25×x10^{-7}\frac{M}{s}}{2}\)
\(\frac{-1.565×10^{-6}\frac{M}{s}}{2}\)
\(-7.83×10^-7\frac{M}{s}\)
Kiwango cha mara moja cha dimerization ni \(-7.83×10^-7\frac{M}{s}\)na vitengo vya kiwango hiki ni \(\frac{M}{s}\).
3.) Kiwango cha wastani cha malezi ya C 8 H 12 saa 1600 s na kiwango cha malezi ya mara moja katika 3200 s kinaweza kupatikana kwa kutumia majibu yetu kutoka sehemu a na b Kama ukiangalia nyuma kwenye equation ya awali, unaweza kuona kwamba C 4 H 6 na C 8 H 12 ni kuhusiana na uwiano mbili hadi moja. Kwa kila moles mbili za C 4 H 6 kutumika, kuna mole moja ya C 8 H 12 zinazozalishwa.
Kwa mmenyuko huu, kiwango cha wastani cha dimerization na kiwango cha wastani cha malezi kinaweza kuunganishwa kupitia usawa huu:
\(\frac{-1}{2}\frac{\Delta [C_4H_6]}{\Delta t}=\frac{\Delta [C_8H_{12}]}{\Delta t}\)
Kumbuka kwamba upande reactant ni hasi kwa sababu reactants ni kuwa kutumika juu katika majibu.
Kwa hiyo, kwa kiwango cha wastani cha malezi ya C 8 H 12 saa 1600 s, tumia kiwango cha dimerization kati ya 0 s na 1600 s tuliyopata mapema na kuziba kwenye equation:
\(\frac{-1}{2}×3.10 × 10^{-6} \frac{M}{s}=\frac{\Delta [C_8H_{12}]}{\Delta t}\)
\(\frac{\Delta [C_8H_{12}]}{\Delta t}=1.55×10^{-6}\frac{M}{s}\)
Kiwango cha wastani cha malezi kwa C 8 H 12 saa 1600 s ni\(1.55×10^{-6}\frac{M}{s}\). Kiwango cha malezi kitakuwa chanya kwa sababu bidhaa zinaundwa.
Kiwango cha malezi ya papo hapo kwa C 8 H 12 kinaweza kuunganishwa na kiwango cha mara moja cha dimerization na equation hii:
\(\frac{-1}{2}\frac{d[C_4H_6]}{dt}=\frac{d[C_8H_{12}]}{dt}\)
Kwa hiyo, kwa kiwango cha malezi ya papo hapo kwa C 8 H 12 saa 3200 s, tumia thamani ya kiwango cha mara moja cha dimerization saa 3200 zilizopatikana mapema na kuziba kwenye equation:
\(\frac{-1}{2}×-7.83×10^-7\frac{M}{s}=\frac{d[C_8H_{12}]}{dt}\)
\(\frac{d[C_8H_{12}]}{dt}=-3.92×10^{-7}\frac{M}{s}\)
Kiwango cha malezi ya papo hapo kwa C 8 H 12 saa 3200 s ni\(-3.92×10^-7\frac{M}{s}\)
- Jibu
-
- \(3.10 × 10^{-6} \frac{M}{s}\)na\(1.04 × 10^{-6} \frac{M}{s}\)
- \(-7.83×10^-7\frac{M}{s}\)na\(\frac{M}{s}\)
- \(-3.92×10^-7\frac{M}{s}\)
Q12.1.5
Utafiti wa kiwango cha majibu kuwakilishwa kama\(2A⟶B\) alitoa data zifuatazo:
| Muda (s) | 0.0 | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 35.0 |
|---|---|---|---|---|---|---|---|
| [A] (M) | 1.00 | 0.952 | 0.625 | 0.465 | 0.370 | 0.308 | 0.230 |
- Kuamua kiwango cha wastani cha kutoweka kwa A kati ya 0.0 s na 10.0 s, na kati ya 10.0 s na 20.0 s.
- Tathmini kiwango cha papo hapo cha kutoweka kwa A saa 15.0 s kutoka kwenye grafu ya muda dhidi ya [A]. Je, ni vitengo vya kiwango hiki?
- Tumia viwango vilivyopatikana katika sehemu (a) na (b) kuamua kiwango cha wastani cha malezi ya B kati ya 0.00 s na 10.0 s, na kiwango cha mara moja cha malezi ya B saa 15.0 s.
- Suluhisho
-
Ulinganifu:\(\frac{-\bigtriangleup A}{\bigtriangleup time}\) na Kiwango=\(\frac{-\bigtriangleup A}{2\bigtriangleup time}=\frac{\bigtriangleup B}{time}\)
Tatua: 1.) Mabadiliko katika A kutoka 0 hadi 10s ni .625-1 =-.375 hivyo\(\frac{-\bigtriangleup A}{\bigtriangleup time}\) =.375/10= 0.0374 m/s
Vile vile, mabadiliko katika A kutoka sekunde 10 hadi 20 ni .370-.625=-.255 hivyo\(\frac{-\bigtriangleup A}{\bigtriangleup time}\) =.255/20-10= 0.0255m/s
2.) Tunaweza kukadiria sheria ya kiwango cha kuchora pointi dhidi ya equations tofauti ili kuamua utaratibu sahihi.
Zero Order:\[\frac{d[A]}{dt}=-k\nonumber \]\[\int_{A_{\circ}}^{A}d[A]=-k\int_{0}^{t}dt\nonumber \]\[[A]=-kt+[A_{\circ}]\nonumber \]
Amri ya kwanza:\[\frac{d[A]}{dt}=-k[A]\nonumber \]\[\int_{A_{\circ}}^{A}\frac{d[A]}{[A]}=-kdt\nonumber \]\[Ln(A)=-kt+Ln(A_{\circ})\nonumber \]
Amri ya Pili:\[\frac{d[A]}{dt}=-k[A]^{2}\nonumber \]\[\int_{A\circ}^{A}\frac{d[A]}{[A]^{2}}=-k\int_{0}^{t}dt\nonumber \]
\[\frac{1}{[A]}=kt+\frac{1}{[A_{\circ}]}\nonumber \]
Sasa kwa kuwa tumepata mstari kutoka kwa kila utaratibu tutapanga pointi vs [A] y-axis, Ln (A) y mhimili, na a 1/ [A] y-mhimili. Kwa namna yoyote ya viwanja ina pointi nyingi za mstari zitatupa wazo nzuri la utaratibu na mteremko utakuwa thamani ya k.
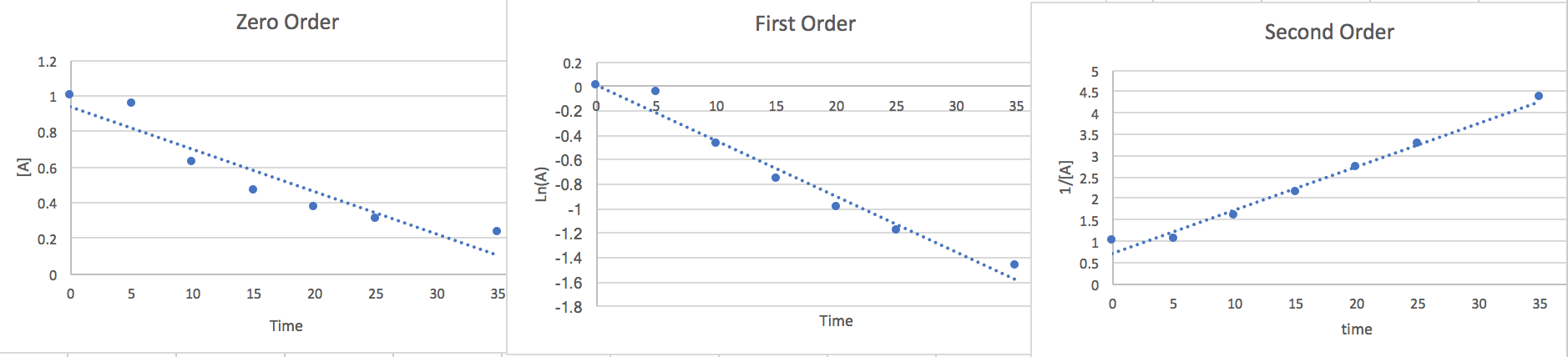
Hapa tunaona kwamba utaratibu wa pili ni wa mstari zaidi hivyo tunahitimisha Kiwango cha kuwa.. \[\frac{-d[A]}{2dt}=k[A]^{2}\nonumber \]Katika sekunde 15 [A] =.465 na kutoka mteremko wa grafu tunapata k=.116.hivyo ikiwa tunaziba data hii ndani na kuzidisha pande zote mbili kwa 2 ili kuondokana na 2 katika denominator upande wa kushoto wa equation tunaona kwamba kiwango cha kutoweka kwa A ni .05 m/s ambapo vitengo ni sawa na [[mol*L -1 *s -1]
3.) Kutumia equation\(\frac{-\bigtriangleup A}{2\bigtriangleup time}=\frac{\bigtriangleup B}{time}\) tunagawanya viwango katika sehemu a na b kwa nusu ili kupata .0188 m/s kutoka sekunde 0 hadi 10 na .025 m/s kwa kiwango cha wastani cha instantaneous saa 15s.
- Jibu
-
(a) kiwango cha wastani, 0 ÷ 10 s = 0.0375 mol L -1 s -1; kiwango cha wastani, 12 - 18 s = 0.0225 mol L -1 s -1; (b) kiwango cha papo hapo, 15 s = 0.0500 mol L -1 s -1; (c) kiwango cha wastani cha malezi ya B = 0.0188 mol L -1 s -1; kiwango cha instantaneous kwa malezi ya B = 0.0250 mol L -1 s -1
Q12.1.6
Fikiria majibu yafuatayo katika suluhisho la maji:
\[\ce{5Br-}(aq)+\ce{BrO3-}(aq)+\ce{6H+}(aq)⟶\ce{3Br2}(aq)+\ce{3H2O}(l)\nonumber \]
Ikiwa kiwango cha kutoweka kwa Br — (aq) kwa wakati fulani wakati wa mmenyuko ni 3.5 × 10 -4 M s -1, ni kiwango gani cha kuonekana kwa Br 2 (aq) wakati huo?
- Suluhisho
-
Hatua ya 1. Eleza kiwango cha majibu.
Kumbuka:
Kwa majibu ya jumla: aA + bB → c+dD
\(rate =- \frac{\Delta[A]}{a\Delta{t}}=- \frac{\Delta[B]}{b\Delta{t}}= \frac{\Delta[C]}{c\Delta{t}}=\frac{\Delta[D]}{d\Delta{t}}\)
Hivyo, kwa majibu:\(5Br^−(aq)+BrO^−_3(aq)+6H^+→3Br_2(aq)+3H_2O(l)\)
Kiwango cha itakuwa:\(rate =- \frac{\Delta[Br^-]}{5\Delta{t}}=- \frac{\Delta[BrO^-_3]}{\Delta{t}}= -\frac{\Delta[H^+]}{6\Delta{t}}=\frac{\Delta[Br_2]}{3\Delta{t}}=\frac{H_2O}{3\Delta{t}}\)
Hatua ya 2. Kwa kuwa sisi ni kupewa kiwango cha upotevu wa\(Br^-\) (aq) ni\(3.5x10^-4 Ms^{-1}\), na tunataka kupata kiwango cha muonekano wa\(Br_2\) (aq). Kwa hiyo sisi kuweka viwango viwili sawa na kila mmoja.
\(rate =- \frac{\Delta[Br^-]}{5\Delta{t}}= \frac{\Delta[Br_2]}{3\Delta{t}}\)
Na,\(-\frac{\Delta[Br^-]}{\Delta{t}}= -3.5x10^{-4} Ms^{-1}\)
Hivyo,\(3.5x10^{-4} Ms^{-1}\) =\(\frac{5}{3}\frac{\Delta[Br_2]}{\Delta{t}}\)
Hatua ya 3. Sasa tatua equation.
\(\frac{(3.5x10^{-4})(3)}{5} = \frac{\Delta[Br_2]}{\Delta{t}}\)
\(\frac{\Delta[Br_2]}{\Delta{t}} = 2.1 x 10^{-4} Ms^{-1}\)
- Jibu
-
\(\frac{\Delta[Br_2]}{\Delta{t}} = 2.1 x 10^{-4} Ms^{-1}\)
12.2: Mambo yanayoathiri Viwango vya Majibu
Q12.2.1
Eleza athari za kila moja ya yafuatayo juu ya kiwango cha mmenyuko wa chuma cha magnesiamu na suluhisho la asidi hidrokloriki: molarity ya asidi hidrokloriki, joto la suluhisho, na ukubwa wa vipande vya magnesiamu.
- Suluhisho
-
Molarity ya Asidi Hidrokloric
- Viwango vya mmenyuko vinaathiriwa na mzunguko ambao molekuli hugongana. High molarity=High Concentration ambayo ina maana molekuli zaidi zinapatikana kwa collide hivyo mmenyuko kasi kwamba moja na molarity chini ya HCl katika kiasi fasta.
- Joto la juu huongeza kiwango cha mmenyuko kwa sababu molekuli huhamia kwa kasi zaidi hivyo kugongana mara nyingi zaidi
- kuongezeka kwa joto kunaruhusu chembe zaidi kuhamia kizuizi cha nishati ya uanzishaji ili kuanza majibu
- kiwango cha mmenyuko unategemea ukubwa imara reactant; vipande vidogo huongeza nafasi ya mgongano kwa sababu wao kuwawezesha zaidi eneo la uso hivyo kasi kiwango cha majibu
Q12.2.2
Nenda kwenye Phet Reactions & Viwango vya maingiliano. Tumia kichupo cha Mgongano mmoja ili kuwakilisha jinsi mgongano kati ya oksijeni ya monatomiki (O) na monoxide ya kaboni (CO) husababisha kuvunja dhamana moja na kuundwa kwa mwingine. Piga nyuma kwenye plunger nyekundu ili uondoe atomi na uangalie matokeo. Kisha, bofya “Reload Launcher” na ubadilishe “Angled risasi” ili uone tofauti.
- Ni nini kinachotokea wakati angle ya mgongano inabadilishwa?
- Eleza jinsi hii inafaa kwa kiwango cha majibu.
- Suluhisho
-
Kulingana na nadharia ya mgongano, kuna mambo mengi yanayosababisha mmenyuko kutokea, huku mambo matatu ni mara ngapi molekuli au atomi hugongana, mwelekeo wa molekuli 'au atomia', na kama kuna nishati ya kutosha kwa ajili ya mmenyuko kutokea. Kwa hiyo, ikiwa pembe ya plunger imebadilishwa, chembe iliyopigwa risasi (chembe moja ya oksijeni katika kesi hii) itapiga molekuli nyingine (CO katika kesi hii) kwa doa tofauti na kwa pembe tofauti, kwa hiyo kubadilisha mwelekeo na idadi ya migongano sahihi haitaweza kusababisha mmenyuko kutokea . Shukrani kwa simulation, tunaweza kuona kwamba hii ni kweli: kulingana na angle iliyochaguliwa, atomi inaweza kuchukua muda mrefu ili kugongana na molekuli na, wakati mgongano unatokea, inaweza kusababisha kuvunja dhamana na kutengeneza nyingine (hakuna majibu hutokea).
Katika hali hii hasa, kiwango cha mmenyuko kitapungua kwa sababu, kwa kubadilisha angle, molekuli au atomi hazitagongana na mwelekeo sahihi au mara nyingi na mwelekeo sahihi.
Q12.2.3
Katika maingiliano ya Phet Reactions & Rates, tumia kichupo cha “Migongano Mingi” kuchunguza jinsi atomi na molekuli nyingi zinavyoingiliana chini ya hali tofauti. Chagua molekuli ya kupiga ndani ya chumba. Weka joto la awali na uchague kiasi cha sasa cha kila reactant. Chagua “Onyesha vifungo” chini ya Chaguzi. Je! Kiwango cha mmenyuko kinaathiriwa na ukolezi na joto?
S12.2.3
Kulingana na Nadharia ya Mgongano, mmenyuko utatokea tu ikiwa molekuli zinakabiliana na mwelekeo sahihi na kwa nishati ya kutosha inayohitajika ili majibu yatoke. Nishati ya chini ambayo molekuli inapaswa kupigana nayo inaitwa nishati ya uanzishaji (nishati ya hali ya mpito).
Kuongezeka kwa mkusanyiko wa reactants huongeza uwezekano kwamba reactants itakuwa collide katika mwelekeo sahihi kwa kuwa kuna reactants zaidi katika kiasi sawa cha nafasi. Kwa hiyo, kuongeza mkusanyiko wa reactants itaongeza kiwango cha mmenyuko. Kupunguza mkusanyiko wa reactants kunapunguza kiwango cha mmenyuko kwa sababu idadi ya jumla ya migongano iwezekanavyo ingepungua.
Joto linahusiana moja kwa moja nishati ya kinetiki ya molekuli na nishati ya uanzishaji\(E_a\) ni nishati ya chini inayohitajika kwa mmenyuko kutokea na haibadiliki kwa mmenyuko. Kuongezeka kwa joto huongeza nishati ya kinetic ya reactants maana ya reactants itahamia kwa kasi na kugongana na kila mmoja mara nyingi zaidi. Kwa hiyo, kuongeza joto huongeza kiwango cha mmenyuko. Kupungua kwa joto hupungua kiwango cha mmenyuko kwani molekuli zitakuwa na nishati ndogo ya kinetic, hoja polepole, na kwa hiyo hugongana na kila mmoja chini ya mara kwa mara.
Q12.2.4
Katika Maingiliano ya Phet & Viwango vya maingiliano, kwenye kichupo cha Migongano Mingi, weka simulation na molekuli 15 za A na molekuli 10 za BC. Chagua “Onyesha vifungo” chini ya Chaguzi.
- Acha Joto la awali kwenye mipangilio ya default. Angalia majibu. Je! Kiwango cha majibu ya haraka au polepole?
- Bonyeza “Pause” halafu “Weka upya Wote,” halafu uingie molekuli 15 za A na molekuli 10 za BC mara nyingine tena. Chagua “Onyesha vifungo” chini ya Chaguzi. Wakati huu, ongezeko joto la awali mpaka, kwenye grafu, mstari wa wastani wa nishati ni juu kabisa ya nguvu ya nishati. Eleza kinachotokea kwa majibu.
- Suluhisho
-
a Katika simulation, sisi kuchagua mazingira default na majibu A+BC. Katika mipangilio ya default, tunaona migongano ya mara kwa mara, joto la chini la awali, na nishati ya wastani ya chini kuliko nishati ya uanzishaji. Nadharia ya mgongano inasema kwamba kiwango cha mmenyuko ni sawia moja kwa moja na (sehemu ya molekuli yenye mwelekeo unaohitajika), (sehemu ndogo za migongano na nishati zinazohitajika), na (mzunguko wa mgongano). Ingawa tunaona kusonga na mara kwa mara kugongana reactants, kiwango cha majibu mbele ni kweli polepole kwa sababu inachukua muda mrefu kwa ajili ya bidhaa, AB na C, kuanza kuonekana. Hii ni hasa kwa sababu sehemu ndogo za migongano na nishati zinazohitajika ni ndogo, zinatokana na nishati ya wastani ya molekuli kuwa chini kuliko nishati ya uanzishaji.
b. majibu yanaendelea kwa kiwango cha kasi zaidi. Tena, nadharia ya mgongano inasema kwamba kiwango cha mmenyuko ni sawia moja kwa moja na (sehemu ya molekuli yenye mwelekeo unaohitajika), (sehemu ndogo za migongano na nishati zinazohitajika), na (mzunguko wa mgongano). Kwa sababu molekuli zina kiasi kikubwa cha nishati, zina nishati zaidi ya kinetic. Kwa nishati ya kinetic iliyoongezeka, molekuli sio tu inagongana zaidi lakini pia huongezeka katika sehemu ya mgongano. Hata hivyo, majibu ya mbele na majibu ya nyuma yanaendelea kwa kiwango cha haraka, hivyo wote hutokea karibu wakati huo huo. Inachukua muda mfupi kwa athari zote mbili kutokea. Pamoja na athari zote mbili zinazoongeza pamoja kwa ujumla, hatimaye kuna hali ya usawa. Mchakato ambao usawa unafikiwa, hata hivyo, ni kasi. Kwa hiyo, kiasi cha bidhaa za A+BC hukaa sawa baada ya muda.
12.3: Sheria za Kiwango
Q12.3.1
Je! Kiwango cha mmenyuko na kiwango chake cha mara kwa mara hutofautiana?
S12.3.1
Kiwango cha majibu au kiwango cha mmenyuko ni mabadiliko katika mkusanyiko wa aidha reactant au bidhaa kwa kipindi cha muda. Ikiwa viwango vinabadilika, kiwango pia kinabadilika.
Kiwango cha A → B:
Kiwango cha mara kwa mara (k) ni mara kwa mara uwiano unaohusiana na viwango vya majibu kwa wahusika. Ikiwa viwango vinabadilika, kiwango cha mara kwa mara hakibadilika.
Kwa mmenyuko na equation ya jumla:\(aA+bB→cC+dD \)
sheria ya kiwango cha majaribio kwa kawaida ina fomu ifuatayo:
Q12.3.2
Mara mbili mkusanyiko wa reactant huongeza kiwango cha mmenyuko mara nne. Kwa ujuzi huu, jibu maswali yafuatayo:
- Je, ni utaratibu gani wa mmenyuko kwa heshima na mtendaji huo?
- Kupunguza mkusanyiko wa reactant tofauti huongeza kiwango cha mmenyuko mara tatu. Je, ni utaratibu gani wa mmenyuko kwa heshima na mtendaji huo?
- Suluhisho
-
(a) 2; (b) 1
Q12.3.3
Kupunguza mkusanyiko wa reactant huongeza kiwango cha mmenyuko mara tisa. Kwa ujuzi huu, jibu maswali yafuatayo:
- Je, ni utaratibu gani wa mmenyuko kwa heshima na mtendaji huo?
- Kuongezeka kwa mkusanyiko wa reactant kwa sababu ya ongezeko nne kiwango cha mmenyuko mara nne. Je, ni utaratibu gani wa mmenyuko kwa heshima na mtendaji huo?
Q12.3.4
Kiasi gani na katika mwelekeo gani kila mmoja yafuatayo itaathiri kiwango cha majibu:\(\ce{CO}(g)+\ce{NO2}(g)⟶\ce{CO2}(g)+\ce{NO}(g)\) ikiwa sheria ya kiwango cha majibu ni\(\ce{rate}=k[\ce{NO2}]^2\)?
- Kupunguza shinikizo la NO 2 kutoka 0.50 atm hadi 0.250 atm.
- Kuongezeka kwa mkusanyiko wa CO kutoka 0.01 M hadi 0.03 M.
- Suluhisho
-
(a) mchakato hupunguza kiwango kwa sababu ya 4. (b) Kwa kuwa CO haionekani katika sheria ya kiwango, kiwango cha si walioathirika.
Q12.3.5
Je, kila moja ya yafuatayo itaathiri kiwango cha mmenyuko:\(\ce{CO}(g)+\ce{NO2}(g)⟶\ce{CO2}(g)+\ce{NO}(g)\) ikiwa sheria ya kiwango cha majibu ni\(\ce{rate}=k[\ce{NO2}][\ce{CO}]\)?
- Kuongezeka kwa shinikizo la NO 2 kutoka 0.1 atm hadi 0.3 atm
- Kuongezeka kwa mkusanyiko wa CO kutoka 0.02 M hadi 0.06 M.
Q12.3.6
Ndege za mara kwa mara za ndege za supersonic katika stratosphere zina wasiwasi kwa sababu ndege hiyo huzalisha oksidi ya nitriki, NO, kama matokeo katika kutolea nje kwa inji zao. Oxydi ya nitriki humenyuka na ozoni, na imependekezwa kuwa hii inaweza kuchangia kupungua kwa safu ya ozoni. Majibu\(\ce{NO + O3⟶NO2 + O2}\) ni amri ya kwanza kuhusiana na NO na O 3 na kiwango cha mara kwa mara cha 2.20 × 10 7 L/mol/s. kiwango instantaneous ya upotevu wa NO wakati [NO] = 3.3 × 10 -6 M na [O 3] = 5.9 × 10 -7 M ?
- Suluhisho
-
4.3 × 10 -5 mol/l/s
Q12.3.7
Fosforasi ya mionzi hutumiwa katika utafiti wa mifumo ya mmenyuko wa biochemical kwa sababu atomi za fosforasi ni sehemu za molekuli nyingi Eneo la fosforasi (na eneo la molekuli linalofungwa) linaweza kuonekana kutoka kwa elektroni (chembe za beta) zinazozalisha:
\[\ce{^{32}_{15}P⟶^{32}_{16}S + e-}\nonumber \]
Kiwango = 4.85 × 10 -1 -2\(\mathrm{day^{-1}\:[^{32}P]}\)
Je, ni kiwango cha instantaneous cha uzalishaji wa elektroni katika sampuli na mkusanyiko wa phosphorus ya 0.0033 M?
Q12.3.8
Kiwango cha mara kwa mara kwa kuoza kwa mionzi ya 14 C ni 1.21 × 10 -4 mwaka -1. Bidhaa za kuoza ni atomi za nitrojeni na elektroni (chembe za beta):
\[\ce{^6_{14}C⟶^{6}_{14}N + e-}\nonumber \]
\[\ce{rate}=k[\ce{^6_{14}C}]\nonumber \]
Ni kiwango gani cha instantaneous cha uzalishaji wa atomi za N katika sampuli na maudhui ya kaboni-14 ya 6.5 × 10 -9 M?
- Suluhisho
-
7.9 × 10 -13 mol/l/mwaka
Q12.3.9
Je, ni kiwango cha instantaneous cha uzalishaji wa atomi N Q12.3.8 katika sampuli yenye maudhui ya kaboni-14 ya 1.5 × 10 -9 M?
Q12.3.10
Uharibifu wa asetaldehyde ni mmenyuko wa pili na kiwango cha mara kwa mara cha 4.71 × 10 -8 L/mol/s. kiwango instantaneous ya kuoza acetaldehyde katika ufumbuzi na mkusanyiko 5.55 × 10 -4 M?
Q12.3.11
Pombe huondolewa kwenye damu kwa mfululizo wa athari za kimetaboliki. Mmenyuko wa kwanza hutoa acetaldehyde; basi bidhaa nyingine zinaundwa. Takwimu zifuatazo zimedhamiriwa kwa kiwango ambacho pombe huondolewa kwenye damu ya mwanamume wastani, ingawa viwango vya mtu binafsi vinaweza kutofautiana kwa 25-30%. Wanawake metabolize pombe polepole zaidi kuliko wanaume:
| [C 2 H 5 OH] (M) | 4.4 × 10 -1 | 3.3 × 10 -2 | 2.2 × 10 -2 |
|---|---|---|---|
| Kiwango (mol/L/h) | 2.0 × 10 —2 | 2.0 × 10 —2 | 2.0 × 10 —2 |
Kuamua kiwango cha equation, kiwango cha mara kwa mara, na utaratibu wa jumla wa mmenyuko huu.
- Suluhisho
-
kiwango = k; k = 2.0 × 10 ÷ 2 Mol/L/h (kuhusu 0.9 g/L/h kwa wanaume wastani); Majibu ni utaratibu wa sifuri.
Q12.3.12
Chini ya hali fulani uharibifu wa amonia kwenye uso wa chuma hutoa data zifuatazo:
| [NH 3] (M) | 1.0 × 10 -3 | 2.0 × 10 -3 | 3.0 × 10 -3 |
|---|---|---|---|
| Kiwango (mol/L/h 1) | 1.5 × 10 -6 | 1.5 × 10 -6 | 1.5 × 10 -6 |
Kuamua kiwango cha equation, kiwango cha mara kwa mara, na utaratibu wa jumla wa mmenyuko huu.
Q12.3.13
Nitrosyl kloridi, NoCl, hutengana na NO na Cl 2.
\[\ce{2NOCl}(g)⟶\ce{2NO}(g)+\ce{Cl2}(g)\nonumber \]
Kuamua kiwango cha equation, kiwango cha mara kwa mara, na utaratibu wa jumla wa majibu haya kutoka data zifuatazo:
| [NoCl] (M) | 0.10 | 0.20 | 0.30 |
|---|---|---|---|
| Kiwango (mol/L/h) | 8.0 × 10 -10 | 3.2 × 10 -9 | 7.2 × 10 -9 |
- Suluhisho
-
Kabla ya tunaweza kufikiri kiwango cha mara kwa mara kwanza ni lazima kwanza kuamua kiwango cha msingi equation na kiwango cha utaratibu. kiwango cha msingi equation kwa mmenyuko huu, ambapo n ni utaratibu wa kiwango cha NoCl na k ni kiwango cha mara kwa mara, ni
\[rate = k[NOCl]^n\nonumber \]
tangu NoCl ni reactant katika mmenyuko.
Ili kufikiri utaratibu wa mmenyuko ni lazima tupate utaratibu wa [NoCl] kama ni reactant pekee katika mmenyuko. Ili kufanya hivyo tunapaswa kuchunguza jinsi kiwango cha mmenyuko kinabadilika kama mkusanyiko wa NoCl unabadilika.
Kama [NoCl] mara mbili katika mkusanyiko kutoka 0.10 M hadi 0.20 M kiwango kinaendelea kutoka 8.0 x 10 -10 hadi 3.2 x 10 -9
(3.2 x 10 -9 (mol/L/h))/(8.0 x 10 -10 (mol/L/h)) = 4
hivyo sisi kuhitimisha kwamba kama [NoCl] mara mbili, kiwango huenda juu kwa 4. Tangu 2 2 = 4 tunaweza kusema kwamba utaratibu wa [NoCl] ni 2 hivyo sheria yetu updated kiwango ni
\[rate = k[NOCl]^2\nonumber \]
Sasa kwa kuwa tuna utaratibu, tunaweza kubadilisha maadili ya kwanza ya majaribio kutoka meza iliyotolewa ili kupata kiwango cha mara kwa mara, k
(8.0 x 10 -10 (mol/l/h)) = k (0.10 M) 2 hivyo
\[k= \dfrac{8.0 \times 10^{-10}}{ (0.10\, M)^2} = 8 \times 10^{-8} M^{-1} sec^{-1}\nonumber \]
Tuliweza kupata vitengo vya k kwa kutumia utaratibu wa kiwango, wakati utaratibu wa kiwango ni 2 vitengo vya k ni M -1 x sec -1
Hivyo kiwango cha equation ni: kiwango = k [NoCl] 2, ni utaratibu wa pili, na k = 8 x 10 -8 M -1 x sec -1
Kwa ujumla kiwango cha sheria:\[rate = \underbrace{(8 \times 10^{-8})}_{\text{1/(M x sec)}} [NOCl]^2\nonumber \]
- Jibu
-
kiwango = k [NoCl] 2; k = 8.0 × 10 -8 L/mol/s; utaratibu wa pili
Q12.3.14
Kutoka data zifuatazo, tambua kiwango cha usawa, kiwango cha mara kwa mara, na utaratibu kwa heshima ya A kwa majibu\(A⟶2C\).
| [A] (M) | 1.33 × 10 -2 | 2.66 × 10 -2 | 3.99 × 10 -2 |
|---|---|---|---|
| Kiwango (mol/L/h) | 3.80 × 10 -7 | 1.52 × 10 -6 | 3.42 × 10 -6 |
- Suluhisho
-
Kutumia data ya majaribio, tunaweza kulinganisha madhara ya kubadilisha [A] juu ya kiwango cha mmenyuko kwa uwiano unaohusiana wa [A] kwa uwiano wa viwango
\[ \frac{2.66 \times 10^{-2}}{1.33 \times 10^{-2}} = 2\nonumber \]na\[ \frac{1.52 \times 10^{-6}}{3.8 \times 10^{-7}} = 4\nonumber \]
B. kutokana na hili tunajua kwamba mara mbili mkusanyiko wa A itasababisha quadrupling kiwango cha majibu. Utaratibu wa mmenyuko huu ni 2.
Sasa tunaweza kuandika kiwango cha equation tangu tunajua ili:
\[rate=k[A]^2\nonumber \]
Kwa kuziba katika seti moja ya data ya majaribio katika kiwango cha equation yetu tunaweza kutatua kwa kiwango cha mara kwa mara, k:
\[3.8 \times 10^{-7} = k \times (1.33 \times 10^{-2})^{2}\nonumber \]
\[k = \frac{3.8 \times 10^{-7}}{1.769 \times 10^{-4}}\nonumber \]
\[k= .00215 M^{-1}s^{-1}\nonumber \]
- Jibu
-
\(k= .00215 M^{-1}s^{-1}\)
Amri ya 2
Q12.3.15
Nitrojeni (II) oksidi humenyuka na klorini kulingana na equation:
\[\ce{2NO}(g)+\ce{Cl2}(g)⟶\ce{2NOCl}(g)\nonumber \]
Viwango vya awali vya majibu yameonekana kwa viwango fulani vya reactant:
| [HAPANA] (Mol/L 1) | [Cl 2] (mol/L) | Kiwango (mol/L/h) |
|---|---|---|
| 0.50 | 0.50 | 1.14 |
| 1.00 | 0.50 | 4.56 |
| 1.00 | 1.00 | 9.12 |
Je, ni kiwango cha equation kinachoelezea utegemezi wa kiwango cha juu ya viwango vya NO na Cl 2? Kiwango cha mara kwa mara ni nini? Je, ni amri gani kwa heshima kwa kila reactant?
- Suluhisho
-
Kwa equation ya jumla,
\(aA + bB \rightarrow cC + dD\)
Kiwango kinaweza kuandikwa kama
\(rate = k[A]^{m}[B]^{n}\)ambapo k ni kiwango cha mara kwa mara, na m na n ni amri ya majibu.
Kwa equation yetu
\(2NO(g) + Cl_{2}(g) \rightarrow 2NOCl(g)\)
ya\(rate = k[NO]^{m}[Cl_{2}]^{n}\)
Sasa, tunahitaji kupata amri za majibu. Maagizo ya majibu yanaweza kupatikana tu kupitia maadili ya majaribio. Tunaweza kulinganisha athari mbili ambapo moja ya reactants ina mkusanyiko sawa kwa majaribio yote, na kutatua kwa utaratibu wa majibu.
\(\frac{rate_{1}}{rate_{2}}=\frac{[NO]_{1}^{m}[Cl_{2}]_{1}^{n}}{[NO]_{2}^{m}[Cl_{2}]_{2}^{n}}\)
Tunaweza kutumia data katika meza iliyotolewa. Kama sisi kuziba katika maadili kwa safu ya 1 na 2, tunaona kwamba maadili kwa mkusanyiko wa Cl kufuta, na kuacha tu viwango na viwango vya NO.
\(\frac{1.14}{4.56}=\frac{[0.5]^{m}}{[1.0]^{m}}\)
Sasa tunaweza kutatua kwa m, na tunaona kwamba m =2. Hii ina maana kwamba ili majibu ya [NO] ni 2.
Sasa ni lazima kupata thamani ya n Kwa kufanya hivyo, tunaweza kutumia equation sawa lakini kwa maadili kutoka safu ya 2 na 3. Wakati huu, mkusanyiko wa NO utaondoa.
\(\frac{4.56}{9.12}=\frac{[0.5]^{n}}{[1.0]^{n}}\)
Wakati sisi kutatua kwa n, tunaona kwamba n = 1. Hii ina maana kwamba ili majibu kwa [Cl 2] ni 1.
Sisi ni hatua moja karibu na kumaliza kiwango cha equation yetu.
\(rate = k[NO]^{2}[Cl_{2}]\)
Hatimaye, tunaweza kutatua kwa kiwango cha mara kwa mara. Ili kufanya hivyo, tunaweza kutumia moja ya majaribio ya jaribio, na kuziba maadili kwa kiwango, na viwango vya reactants, kisha kutatua kwa k.
\(1.14 mol/L/h = k[0.5 mol/L]^{2}[0.5mol/L]\)
\(k=9.12L^{2}mol^{-2}h^{-1}\)
Kwa hiyo, kiwango cha mwisho cha equation ni:
\(rate = (9.12 L^{2} mol^{-2}h^{-1})[NO]^{2}[Cl_{2}]\)
* Hitilafu ya kawaida ni kusahau vitengo. Hakikisha kufuatilia vitengo yako katika mchakato wa kuamua kiwango chako mara kwa mara. Kuwa makini kwa sababu vitengo vitabadilika kuhusiana na utaratibu wa majibu.
- Jibu
-
kiwango = k [NO] 2 [Cl] 2; k = 9.12 L 2 mol -2 h -1; utaratibu wa pili katika NO; utaratibu wa kwanza katika Cl 2
Q12.3.17
Hidrojeni humenyuka na monoksidi nitrojeni kuunda monoksidi ya dinitrojeni (gesi ya kucheka) kadiri ya mlinganyo
\[\ce{H2}(g)+\ce{2NO}(g)⟶\ce{N2O}(g)+\ce{H2O}(g)\nonumber \]
Kuamua kiwango cha equation, kiwango cha mara kwa mara, na amri kwa heshima kwa kila reactant kutoka data zifuatazo:
| [HAPANA] (M) | 0.30 | 0.60 | 0.60 |
|---|---|---|---|
| [H 2] (M) | 0.35 | 0.35 | 0.70 |
| Kiwango (mol/l/s) | 2.835 × 10 -3 | 1.134 × 10 -2 | 2.268 × 10 -2 |
- Suluhisho
-
Kuamua kiwango cha equation, kiwango cha mara kwa mara, na amri kwa heshima na kila reactant.
Kiwango cha mara kwa mara na amri zinaweza kuamua kupitia sheria ya kiwango cha tofauti. Fomu ya jumla ya sheria ya kiwango cha tofauti hutolewa hapa chini:
Aa + bB + cc => bidhaa
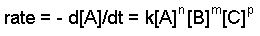
ambapo A, B, na C ni viwango vya reactants, k ni kiwango cha mara kwa mara, na n, m, na p hutaja utaratibu wa kila reactant.
Ili kupata maagizo ya kila reactant, tunaona kwamba wakati [NO] mara mbili lakini [H 2] haibadiliki, kiwango cha quadruples, maana kwamba [NO] ni mmenyuko wa utaratibu wa pili ([NO] 2). Wakati [H 2] mara mbili lakini [NO] haibadiliki, kiwango kinaongezeka mara mbili, maana yake ni kwamba [H 2] ni mmenyuko wa utaratibu wa kwanza. Hivyo sheria ya kiwango bila kuangalia kitu kama hiki:
Kiwango = k [NO] 2 [H 2]
Tunaweza kutumia sheria hii ya kiwango kuamua thamani ya kiwango cha mara kwa mara. Kuziba katika data kwa mkusanyiko reactant na kiwango kutoka moja ya majaribio ya kutatua kwa k kiwango cha mara kwa mara. Katika kesi hii, tulichagua kutumia data kutoka kwa jaribio 1 kutoka safu ya pili ya meza ya data.
2.835x10 -3 = k [0.3] 2 [0.35]
k = 0.9 M -2 /s -1
Q12.3.18
Kwa majibu\(A⟶B+C\), data zifuatazo zilipatikana saa 30 °C:
| [A] (M) | 0.230 | 0.356 | 0.557 |
|---|---|---|---|
| Kiwango (mol/l/s) | 4.17 × 10 -4 | 9.99 × 10 -4 | 2.44 × 10 -3 |
- Je, ni utaratibu gani wa mmenyuko kwa heshima na [A], na ni kiwango gani cha equation?
- Kiwango cha mara kwa mara ni nini?
- Suluhisho
-
1. kiwango equation kwa majibu\(n\) ili ni kutolewa kama\(\frac{dr}{dt}={k}{[A]^n}\). Ambapo\([A]\) ni mkusanyiko katika M, na\(\frac{dr}{dt}\) ni kiwango cha m/s.
Tunaweza kisha kutumia kila seti ya pointi data, kuziba maadili yake katika equation kiwango na kutatua kwa\(n\). Kumbuka: unaweza kutumia pointi yoyote ya data kwa muda mrefu kama ukolezi unafanana na kiwango chake.
Kiwango cha usawa 1:\(4.17 \times {10}^{-4}={k}{[0.230]^n}\)
Kiwango cha usawa 2:\(9.99 \times {10}^{-4}={k}{[0.356]^n}\)
Tunagawanya Kiwango cha equation 1 na Kiwango cha equation 2 ili kufuta k, kiwango cha mara kwa mara.
\({\frac{4.17 \times {10}^{-4}}{9.99 \times {10}^{-4}}} = {\frac{k[0.230]^n}{k[0.356]^n}} \)
\({0.417}={0.646^n}\)
Sasa haijulikani tu tunayo ni\(n\). Kutumia sheria za logarithm mtu anaweza kutatua.
\(ln{\: 0.417}={n \cdot ln{\: 0.646}}\)
\(\frac{ln{\: 0.417}}{ln{\:0.646}}=n=2\)
kiwango equation ni utaratibu wa pili kwa heshima na A na imeandikwa kama\(\frac{dr}{dt}={k}{[A]^2}\).
2. Tunaweza kutatua\(k\) kwa kuziba katika hatua yoyote data katika kiwango cha equation yetu\(\frac{dr}{dt}={k}{[A]^2}\).
Kutumia pointi za kwanza za data kwa mfano\( [A]=0.230 \:\frac{mol}{L}\) na\( \frac{dr}{dt} = 4.17 \times {10}^{-4} \:\frac{mol}{L \cdot s}\)] tunapata equation\(4.17 \times {10}^{-4} \:\frac{mol}{L \cdot s}={k}{[0.230 \:\frac{mol}{L}]^2}\)
Ambayo hutatua\(k=7.88 \times {10}^{-3} \frac{L}{mol \cdot s}\)
Kwa kuwa tunajua hii ni majibu ya utaratibu wa pili, vitengo vinavyofaa\(k\) vinaweza pia kuandikwa kama\( \frac{1}{M \cdot s}\)
- Jibu
-
(a) kiwango equation ni utaratibu wa pili katika A na imeandikwa kama kiwango = k [A] 2. (b) k = 7.88 × 10 -13 L mol -1 s -1
Q12.3.19
Kwa majibu\(Q⟶W+X\), data zifuatazo zilipatikana saa 30 °C:
| [Q] awali (M) | 0.170 | 0.212 | 0.357 |
|---|---|---|---|
| Kiwango (mol/l/s) | 6.68 × 10 -3 | 1.04 × 10 —2 | 2.94 × 10 —2 |
- Je, ni utaratibu gani wa mmenyuko kwa heshima na [Q], na ni kiwango gani cha equation?
- Kiwango cha mara kwa mara ni nini?
- Suluhisho
-
Je, ni utaratibu gani wa mmenyuko kwa heshima na [Q], na ni kiwango gani cha equation?
- Amri ya majibu: 2 kwa sababu unapotumia jaribio la uwiano 3:2, litaonekana kama hii:
- (\(\dfrac{2.94*10^{-2}}{1.04*10^{-2}}\)) = (\(\dfrac{0.357^{x}}{0.212^{x}}\))
- 2.82 = 1.7 x
- x = 2 hivyo utaratibu wa majibu ni 2
- Kiwango cha mmenyuko equation: Kiwango=K [Q] 2
- Ili kupata kiwango cha mara kwa mara (k) tu kuziba na kuhesabu moja ya majaribio katika equation kiwango
- 1.04 x 10 -2 =k [0.212] 2
- k=0.231\(M^{-1}s^{-1}\)
- Jibu
-
Order: 2
k=0.231\(M^{-1}s^{-1}\)
Q12.3.20
Kiwango cha mara kwa mara kwa utengano wa kwanza wa 45° C ya pentoxide ya dinitrojeni, N 2 O 5, kufutwa katika klorofomu, ChCl 3, ni 6.2 × 10 -4 min -1.
\[\ce{2N2O5⟶4NO2 + O2}\nonumber \]
Je, ni kiwango gani cha mmenyuko wakati [N 2 O 5] = 0.40 M?
- Suluhisho
-
Hatua ya 1: Hatua ya kwanza ni kuandika sheria ya kiwango. Tunajua formula ya jumla kwa sheria ya kiwango cha kwanza ili. Ni kama ifuatavyo: rate=K [A]
Hatua ya 2: Sasa tunaziba [N 2 the 5] kwa [A] katika sheria yetu ya kiwango cha jumla. Sisi pia kuziba katika kiwango cha mara kwa mara yetu (k), ambayo tulipewa. Sasa equation yetu inaonekana kama ifuatavyo:
Kiwango = (6.2x10 -4 min -1) [N 2 O 5]
Hatua ya 3: Sasa tunaziba katika molarity yetu iliyotolewa. [N 2 O 5] =0.4 M. sasa equation yetu inaonekana kama ifuatavyo:
Kiwango = (6.2x10 -4 min -1) (0.4 M)
Hatua ya 4: Sasa tunatatua equation yetu. Kiwango = (6.2x10 -4 min -1) (0.4 M) = 2.48x10 -4 m/min.
Hatua ya 5: Tumia takwimu muhimu na uongofu wa kitengo kwa pande zote 2.48x10 -4 m/min hadi 2.5 × 10 -4 (moles) L -1 min -1
- Jibu
-
(a) 2.5 × 10 -4 mol/L/min
Q12.3.21
Uzalishaji wa kila mwaka wa HNO 3 katika 2013 ulikuwa tani milioni 60 Zaidi ya hayo iliandaliwa na mlolongo wa athari zifuatazo, kila kukimbia katika chombo tofauti majibu.
- \(\ce{4NH3}(g)+\ce{5O2}(g)⟶\ce{4NO}(g)+\ce{6H2O}(g)\)
- \(\ce{2NO}(g)+\ce{O2}(g)⟶\ce{2NO2}(g)\)
- \(\ce{3NO2}(g)+\ce{H2O}(l)⟶\ce{2HNO3}(aq)+\ce{NO}(g)\)
Menyu ya kwanza inaendeshwa na kuchomwa amonia katika hewa juu ya kichocheo cha platinamu. Majibu haya ni ya haraka. Majibu katika equation (c) pia ni ya haraka. Mmenyuko wa pili hupunguza kiwango ambacho asidi ya nitriki inaweza kuandaliwa kutoka kwa amonia. Ikiwa equation (b) ni utaratibu wa pili katika NO na utaratibu wa kwanza katika O 2, ni kiwango gani cha malezi ya NO 2 wakati mkusanyiko wa oksijeni ni 0.50 M na mkusanyiko wa oksidi ya nitriki ni 0.75 M? Kiwango cha mara kwa mara kwa majibu ni 5.8 × 10 -6 L 2 /mol 2 /s.
- Suluhisho
-
Kuamua sheria ya kiwango cha equation tunahitaji kuangalia hatua yake ya polepole. Kwa kuwa equation zote a na c ni za haraka, equation b inaweza kuchukuliwa hatua ya polepole ya majibu. Hatua ya polepole pia inachukuliwa kuwa kiwango cha kuamua hatua ya mfumo.
Hivyo, kiwango cha kuamua hatua ni hatua ya pili kwa sababu ni hatua polepole.
kiwango cha uzalishaji wa\(NO_2 = k [A]^m [B]^n \)
\(rate = k [NO]^2 [O_2]^1~M/s\)
\(rate = (5.8*10^{-6}) [0.75]^2 [0.5]^1 ~M/s\)
\(rate = 1.6*10^{-6}~M/s\)
- Jibu
-
\(rate = 1.6*10^{-6}~M/s\)
Q12.3.22
Takwimu zifuatazo zimewekwa kwa majibu:
\[\ce{I- + OCl- ⟶ IO- + Cl-}\nonumber \]
| 1 | 2 | 3 | |
|---|---|---|---|
| \(\mathrm{[I^-]_{initial}}\)(M) | 0.10 | 0.20 | 0.30 |
| \(\mathrm{[OCl^-]_{initial}}\)(M) | 0.050 | 0.050 | 0.010 |
| Kiwango (mol/l/s) | 3.05 × 10 -4 | 6.20 × 10 -4 | 1.83 × 10 -4 |
Kuamua kiwango cha equation na kiwango cha mara kwa mara kwa mmenyuko huu.
- Suluhisho
-
Kutumia reactants, tunaweza kuunda sheria ya kiwango cha majibu: $ r=k [OCL^-] ^n [I^-] ^m\]
Kutoka huko, tunahitaji kutumia data ili kuamua utaratibu wa wote\([OCl^-]\) na\([I^-]\). Kwa kufanya hivyo, tunahitaji kulinganisha\(r_1\) na\(r_2\) vile vile:
\[ \frac {r_1}{r_2} = \frac {(0.10^m)(0.050^n)}{(0.20^m)(0.050^n)} = \frac {3.05 \times 10^{-4}}{6.20 \times 10^{-4}} \]
\[ 0.5^m = 0.5 \]
\[ m = 1 \]
Tunaweza “kuvuka nje” mkusanyiko wa\([OCl^-]\) sababu ina mkusanyiko huo katika wote wa majaribio kutumika.
Sasa kwa kuwa tunajua m (\([I^-]\)) ina utaratibu wa kwanza wa 1.
Hatuwezi “kuvuka nje”\([I^-]\) ili kupata\([OCl^-]\) kwa sababu hakuna majaribio mawili yaliyo na ukolezi sawa. Ili kutatua kwa n tutaziba 1 kwa m.
\[ \frac {r_1}{r_3} = \frac {(0.10^{1})(0.050^n)}{(0.30^{1})(0.010^n)} = \frac {3.05 \times 10^{-4}}{1.83 \times 10^{-4}} \]
\[ \frac {1}{3} (5^{n}) = 1.6666667 \]
\[ 5^{n} = 5 \]
\[ n = 1 \]
Kwa kuwa tunajua kwamba maagizo ya n na m wote ni sawa na moja, hatuwezi badala yao katika kiwango cha sheria equation pamoja na viwango husika (kutoka aidha ya kwanza, ya pili, au ya tatu majibu) na kutatua kwa kiwango cha mara kwa mara, k.
\[ r=k[OCl^-]^n[I^-]^m \]
\[ 3.05 * 10^{-4}= k[0.05]^1[0.10]^1 \]
\[ k = 6.1 * 10^{-2} \frac {L}{mol \times s} \]
Hivyo sheria ya kiwango cha jumla ni: $ r = (6.1 * 10^ {-2}\ frac {L} {mol\ mara s}) [OCL^-] [I^-]\]
Vitengo vya K hutegemea utaratibu wa jumla wa majibu. Ili kupata utaratibu wa jumla tunaongeza m na n pamoja. Kwa kufanya hivyo tunapata utaratibu wa jumla wa 2. Hii ni kwa nini vitengo kwa K ni $\ frac {L} {mol\ mara s}\]
- Jibu
-
kiwango = k [I -] [Ocl -1]; k = 6.1 × 10 -2 L mol -1 s -1
Q12.3.23
Katika mmenyuko
\[2NO + Cl_2 → 2NOCl\nonumber \]
reactants na bidhaa ni gesi katika joto la mmenyuko. Takwimu zifuatazo za kiwango zilipimwa kwa majaribio matatu:
| Awali p {NO} | Awali p {Cl 2} | Kiwango cha awali |
|---|---|---|
| (atm) | (atm) | (moles ya sekunde ya atm iliyotumiwa -1) |
| 0.50 | 0.50 | 5.1 x 10 -3 |
| 1.0 | 1.0 | 4.0 x 10 -2 |
| 0.50 | 1.0 | 1.0 x 10 -2 |
- Kutoka data hizi, andika equation ya kiwango kwa mmenyuko huu wa gesi. Nini utaratibu ni majibu katika NO, Cl 2, na kwa ujumla?
- Tumia kiwango cha mara kwa mara kwa mara kwa mmenyuko huu.
- Suluhisho
-
a. kiwango equation inaweza kuamua na kubuni majaribio kwamba kupima mkusanyiko (s) ya reactants moja au zaidi au bidhaa kama kazi ya muda. Kwa majibu\(A+B\rightarrow products\), kwa mfano, tunahitaji kuamua k na exponents m na n katika equation ifuatayo: Kwa
\[rate=k[A]^m[B]^n\nonumber \]
kufanya hivyo, mkusanyiko wa awali wa B unaweza kuhifadhiwa mara kwa mara wakati tofauti ukolezi wa awali ya A na kuhesabu kiwango cha awali mmenyuko. Habari hii bila kubainisha ili majibu kuhusiana na A. mchakato huo unaweza kufanyika ili kupata ili majibu
kuhusiana na B. mfano huu,
\[\frac{rate_2}{rate_3}=\frac{k[A_2]^m[B_2]^n}{k[A_3]^m[B_3]^n}\nonumber \]
Hivyo kuchukua maadili kutoka meza,
\[\frac{4.0*10^{-2}}{1.0*10^{-2}}=\frac{k[1.0]^m[1.0]^n}{k[0.5]^m[1.0]^n}\nonumber \]
na kwa kufuta kama maneno, umesalia na
\[\frac{4.0*10^{-2}}{1.0*10^{-2}}=\frac{[1.0]^m}{[0.5]^m}\nonumber \]
Sasa, tatua kwa m
\(4=2^m\Longrightarrow m=2\) Kwa sababu m = 2, majibu kwa heshima\(NO\) ni 2. \(NO\)ni utaratibu wa pili.
Unaweza kurudia mchakato huo ili kupata n
\[\frac{rate_3}{rate_1}=\frac{k[A_3]^m[B_3]^n}{k[A_1]^m[B_1]^n}\nonumber \]
Kuchukua maadili kutoka meza,
\[\frac{1.0*10^{-2}}{5.1*10^{-3}}=\frac{k[0.5]^m[1.0]^n}{k[0.5]^m[0.5]^n}\nonumber \]
na kwa kufuta kama maneno, wewe ni wa kushoto na
\[\frac{1.0*10^{-2}}{5.1*10^{-3}}=\frac{[1.0]^n}{[0.5]^n}\nonumber \]
Sasa wakati huu, kutatua kwa n
\(2=2^n\Longrightarrow n=1\)
Kwa sababu n = 1, majibu kwa heshima na\(Cl_2\) ni 1. \(Cl_2\)ni utaratibu wa kwanza.
Hivyo kiwango cha equation ni\[rate=k[NO]^2[Cl_2]^1\nonumber \]
Kupata utaratibu wa jumla kiwango, wewe tu kuongeza maagizo pamoja. Ili pili + utaratibu wa kwanza hufanya majibu ya jumla ili tatu.b. kiwango cha mara kwa mara ni mahesabu kwa kuingiza data kutoka mstari wowote wa meza katika sheria ya kiwango cha majaribio na kutatua kwa k Kwa majibu ya utaratibu wa tatu, vitengo vya k ni\(frac{1}{atm^2*sec}\). Kutumia majaribio 1,
\[rate=k[NO]^2[Cl_2]^1\Longrightarrow 5.1*10^{-3} \frac{atm}{sec}=k[0.5m atm]^2[0.5 atm]^1\nonumber \]
\[k=0.0408 \frac{1}{atm^2*sec}\nonumber \]
- Jibu
-
\(NO\)ni utaratibu wa pili.
\(Cl_2\)ni utaratibu wa kwanza.
Kwa ujumla ili majibu ni tatu.
b)
\(k=0.0408\; atm^{-2}*sec^{-1}\)
12.4: Sheria za Kiwango cha Jumuishi
Q12.4.1
Eleza jinsi mbinu za kielelezo zinaweza kutumiwa kuamua utaratibu wa mmenyuko na kiwango chake cha mara kwa mara kutoka kwa mfululizo wa data inayojumuisha mkusanyiko wa A kwa nyakati tofauti.
- Suluhisho
-
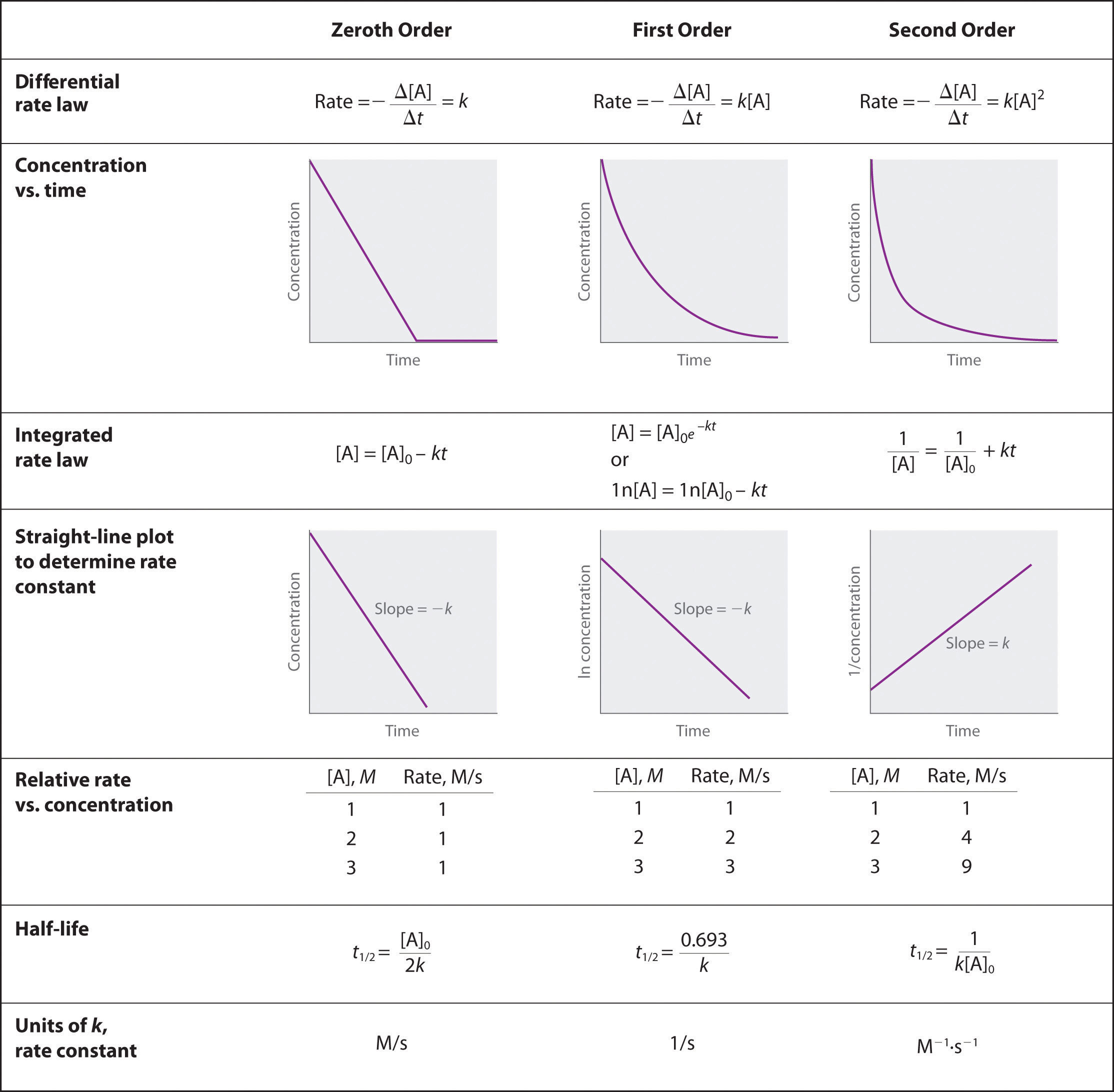
Kuamua utaratibu wa mmenyuko unapopewa mfululizo wa data, mtu lazima apate graph data jinsi ilivyo, grafu kama logi ya asili ya [A], na kuiweka kama 1/ [A]. Njia yoyote inazalisha mstari wa moja kwa moja itaamua utaratibu. Sambamba ya mbinu za kuchora hapo juu, ikiwa mstari wa moja kwa moja unatolewa na njia ya kwanza ya kuchora ni utaratibu wa 0, ikiwa kwa njia ya pili ni utaratibu wa 1, na njia ya tatu ya kuchora ni utaratibu wa 2. Wakati utaratibu wa grafu unajulikana, mfululizo wa milinganyo, iliyotolewa katika picha hapo juu, inaweza kutumika kwa pointi mbalimbali kwenye grafu kuamua thamani ya k Tunaweza kuona kwamba tunahitaji thamani ya awali ya A na thamani ya mwisho ya A, na wote hawa watapewa na data.
Ili Zeroth wakati wa kupanga mipango ya awali ya kuzingatia dhidi ya ukolezi wa mwisho una mteremko usiofaa wa mstari.
\[[A] = [A]_0 − kt\nonumber \]
Kwanza ili wakati wa kupanga njama juu ya [mkusanyiko wa awali] dhidi ya ln [ukolezi wa mwisho] una mteremko hasi linear.
\[\ln[A] = \ln[A]_0 − kt\nonumber \]
Mpangilio wa pili wakati wa kupanga mpango wa 1/ [mkusanyiko wa awali] dhidi ya 1/ [ukolezi wa mwisho] una mteremko mzuri wa mstari.
\[\dfrac{1}{[\textrm A]}=\dfrac{1}{[\textrm A]_0}+kt\nonumber \]
Q12.4.2
Tumia data iliyotolewa kwa graphically kuamua utaratibu na kiwango cha mara kwa mara ya majibu yafuatayo:\(\ce{SO2Cl2 ⟶ SO2 + Cl2}\)
| Muda (s) | 0 | 5.00 × 10 3 | 1.00 × 10 4 | 1.50 × 10 4 | 2.50 × 10 4 | 3.00 × 10 4 | 4.00 × 10 4 |
|---|---|---|---|---|---|---|---|
| [SO 2 Cl 2] (M) | 0.100 | 0.0896 | 0.0802 | 0.0719 | 0.0577 | 0.0517 | 0.0415 |
- Suluhisho
-
Tumia data ili ueleze utaratibu na kiwango cha mara kwa mara cha majibu yafuatayo.
Ili kuamua kiwango cha sheria kwa majibu kutoka seti ya data yenye mkusanyiko (au maadili ya baadhi ya kazi ya mkusanyiko) dhidi ya muda, kufanya grafu tatu za data kulingana na sheria jumuishi kiwango cha kila mmenyuko ili.
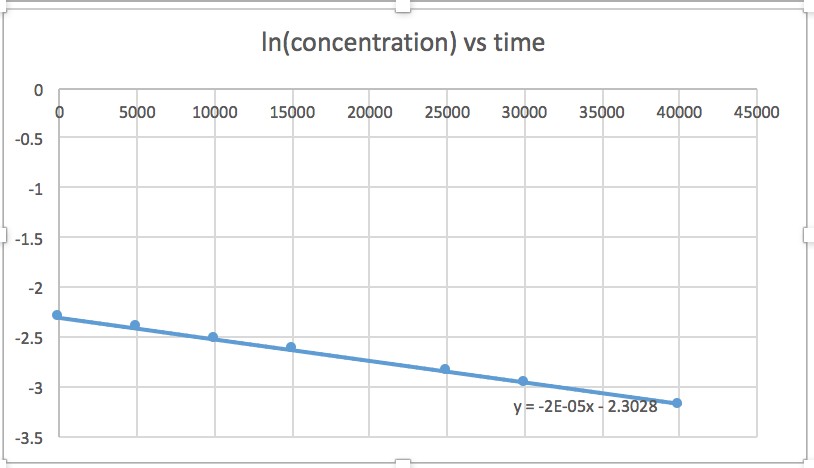
[mkusanyiko] dhidi ya muda (linear kwa majibu ili sifuri) ln [mkusanyiko] dhidi ya wakati (linear kwa 1 st ili majibu) 1/[mkusanyiko] dhidi ya muda (linear kwa 2 nd ili majibu)mteremko = -2.0 x 10 -5
k = 2.0 x 10 -5
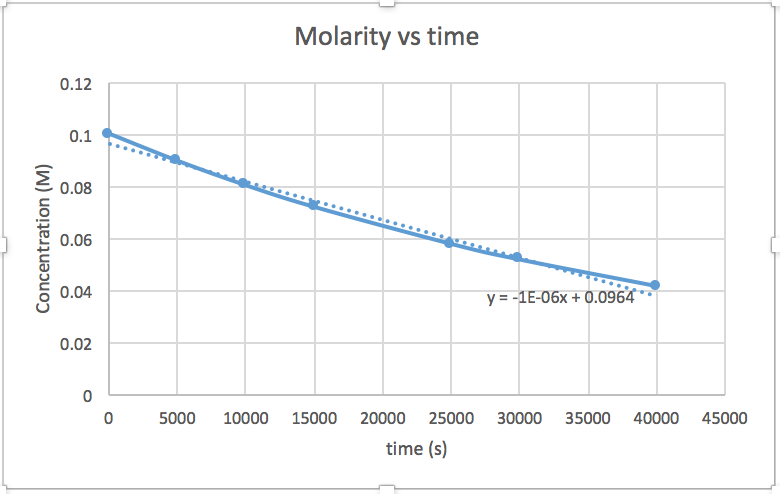
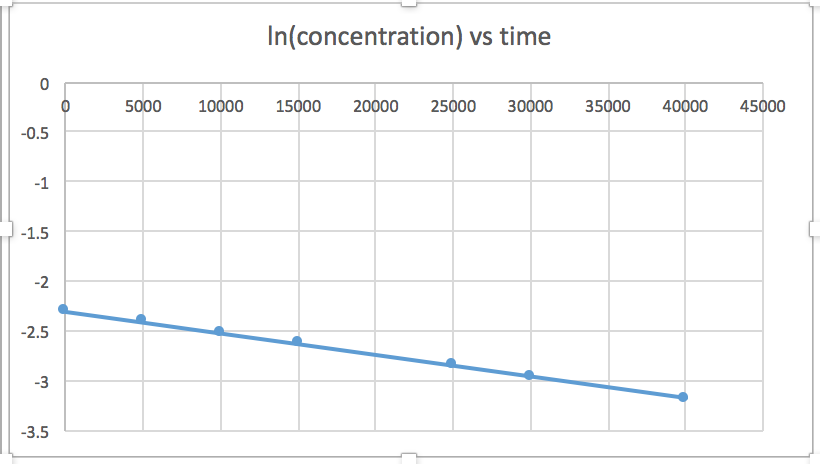
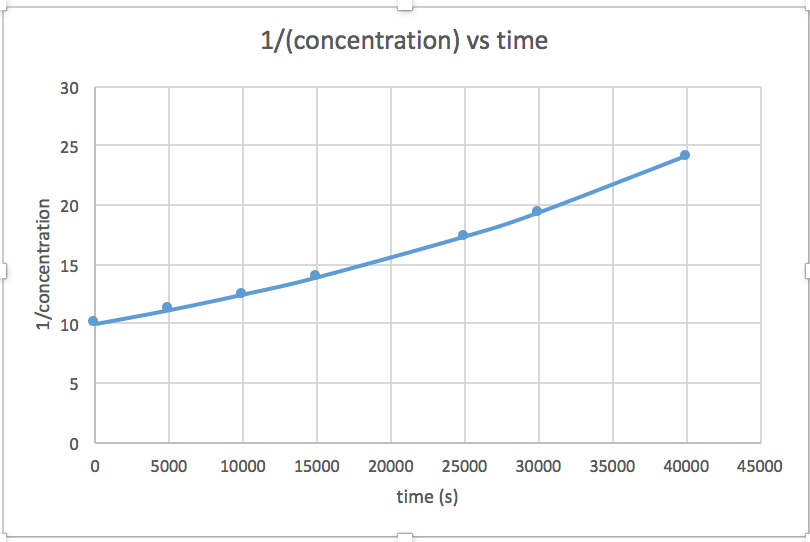
Grafu ambayo ni mstari inaonyesha utaratibu wa majibu. Kisha, unaweza kupata usawa wa kiwango sahihi:
mmenyuko wa utaratibu wa sifuri kiwango = k (k = - mteremko wa mstari) 1 mmenyuko wa utaratibu kiwango = k [A] (k = - mteremko wa mstari) 2 nd ili majibu kiwango = k [A] 2 (k = mteremko wa mstari) Katika grafu hii, ln (mkusanyiko) vs wakati ni linear, kuonyesha kwamba majibu ni ya kwanza.
k=-mteremko wa mstari
- Jibu
-
Kupanga grafu ya ln [SO 2 Cl 2] dhidi ya t inaonyesha mwenendo linear; kwa hiyo tunajua hii ni majibu ya kwanza:
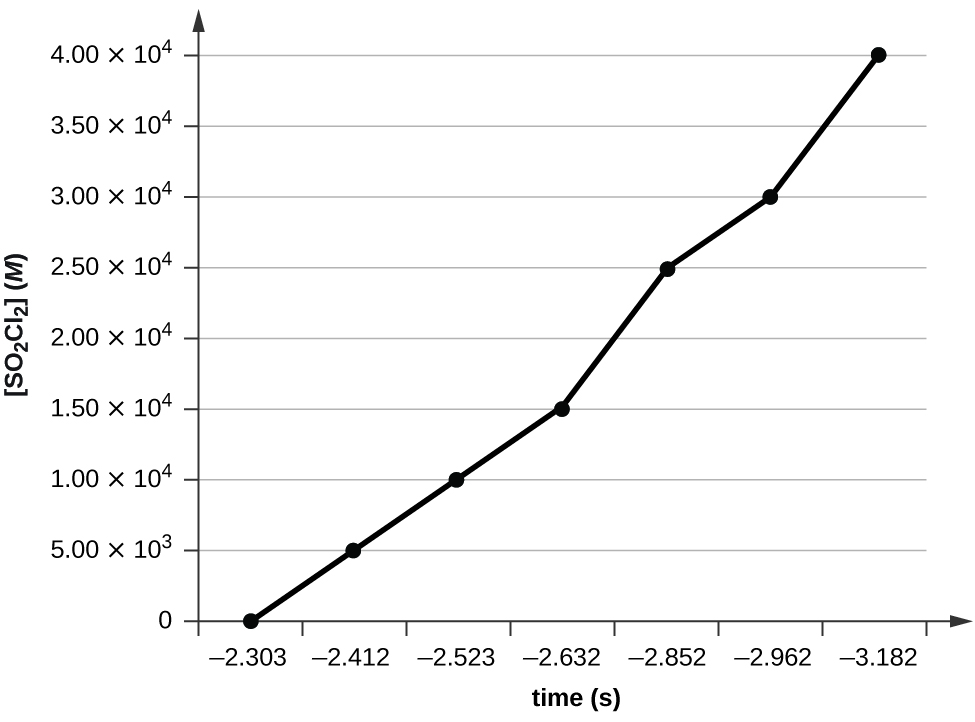
k = -2.20 × 10 5 s -1
Q12.4.3
Tumia data iliyotolewa kwa njia ya kielelezo ili kuamua utaratibu na kiwango cha mara kwa mara ya majibu yafuatayo:
\[2P⟶Q+W\nonumber \]
| Muda (s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
|---|---|---|---|---|---|
| [P] (M) | 1.077 × 10 -3 | 1.068 × 10 -3 | 1.055 × 10 -3 | 1.046 × 10 -3 | 1.039 × 10 -3 |
- Suluhisho
-
Ongeza maandiko hapa. Usifute maandishi haya kwanza.
Q12.4.4
Ozoni safi hutengana polepole kwa oksijeni,\(\ce{2O3}(g)⟶\ce{3O2}(g)\). Tumia data iliyotolewa kwa njia ya kielelezo na ueleze utaratibu na kiwango cha mara kwa mara cha majibu.
| Muda (h) | 0 | 2.0 × 10 3 | 7.6 × 10 3 | 1.23 × 10 4 | 1.70 × 10 4 | 1.70 × 10 4 |
|---|---|---|---|---|---|---|
| [O 3] (M) | 1.00 × 10 -5 | 4.98 × 10 -6 | 2.07 × 10 -6 | 1.39 × 10 -6 | 1.22 × 10 -6 | 1.05 × 10 -6 |
- Suluhisho
-
Kuamua utaratibu na kiwango cha mara kwa mara, unahitaji kuchora data kwa utaratibu wa sifuri, utaratibu wa kwanza, na utaratibu wa pili kwa kupanga mipango dhidi ya wakati- [A] vs. wakati, logarithm ya asili (ln) ya [A] vs wakati, na 1/ [A] vs wakati kwa mtiririko huo. Utaratibu wa mmenyuko umeamua kwa kutambua ni ipi kati ya grafu hizi tatu hutoa mstari wa moja kwa moja. Kiwango cha mara kwa mara k kinawakilishwa na mteremko wa grafu. Grafu na maadili yao ya data itakuwa
Muda (s) 9.0 13.0 18.0 22.0 25.0 [P] (M) 1.077 × 10—3 1.068 × 10—3 1.055 × 10—3 1.046 × 10—3 1.039 × 10—3 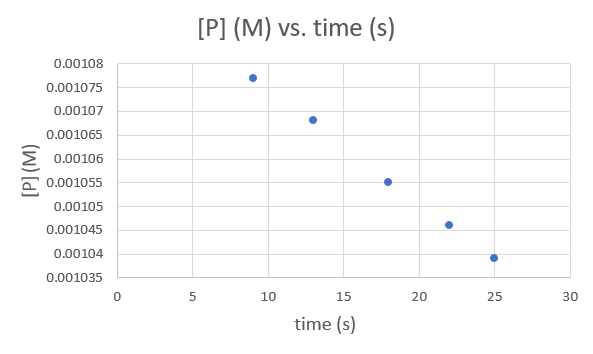.png)
Muda (s) 9.0 13.0 18.0 22.0 25.0 ln [L] (M) - 6.83358 -6.84197 -6.85421 -6.86278 -6.8695 .png)
Muda (s) 9.0 13.0 18.0 22.0 25.0 1/ [P] (M) 928.5051 936.3296 947.8673 956.0229 962.4639 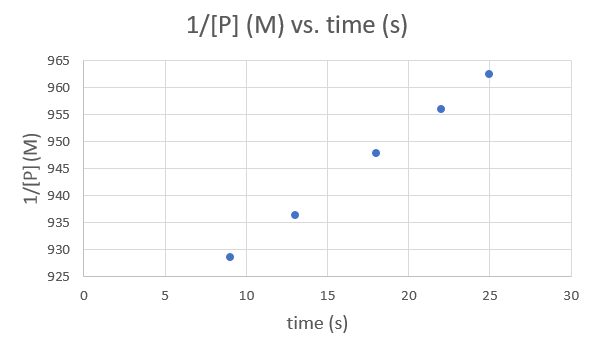.png)
Kwa kuwa kila grafu hutoa mstari wa moja kwa moja utaratibu na kiwango cha mara kwa mara ya mmenyuko hauwezi kuamua.
Ili kutambua jinsi viwango vinavyobadilika kazi ya muda, inahitaji kutatua usawa wa kutofautisha sahihi (yaani, sheria ya kiwango cha tofauti).
Sheria ya kiwango cha sifuri inabiri katika kuoza kwa mstari wa mkusanyiko na
wakati. sheria ya kiwango cha kwanza cha utaratibu inabiri katika kuoza kielelezo kwa ukolezi
kwa wakati Sheria ya kiwango cha 2 ili inabiri katika kuoza kwa usawa wa mkusanyiko na wakati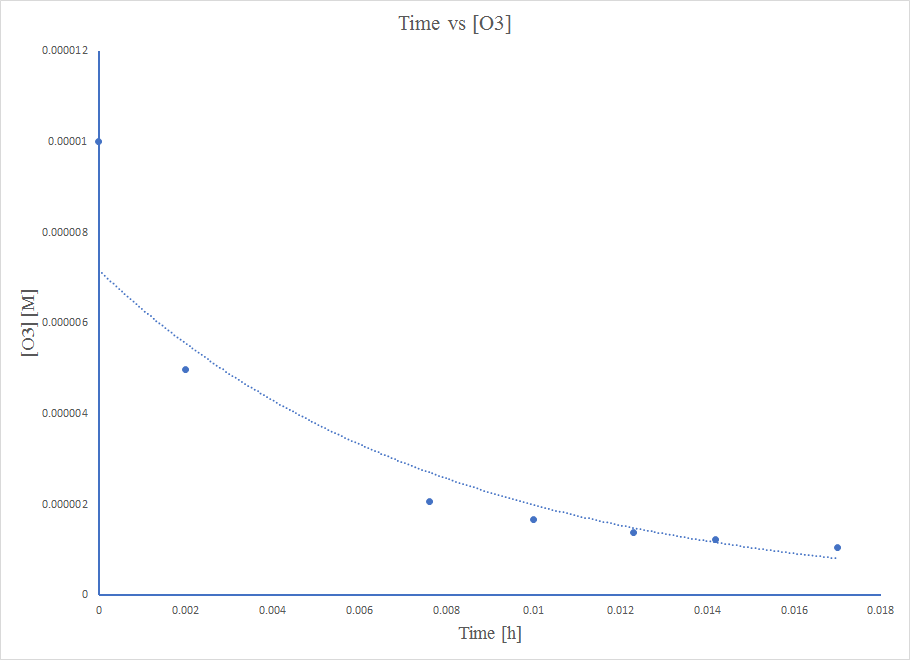
Mpango huo sio mstari, hivyo majibu sio utaratibu wa sifuri.

Mpango huo sio mstari, hivyo majibu sio utaratibu wa kwanza.
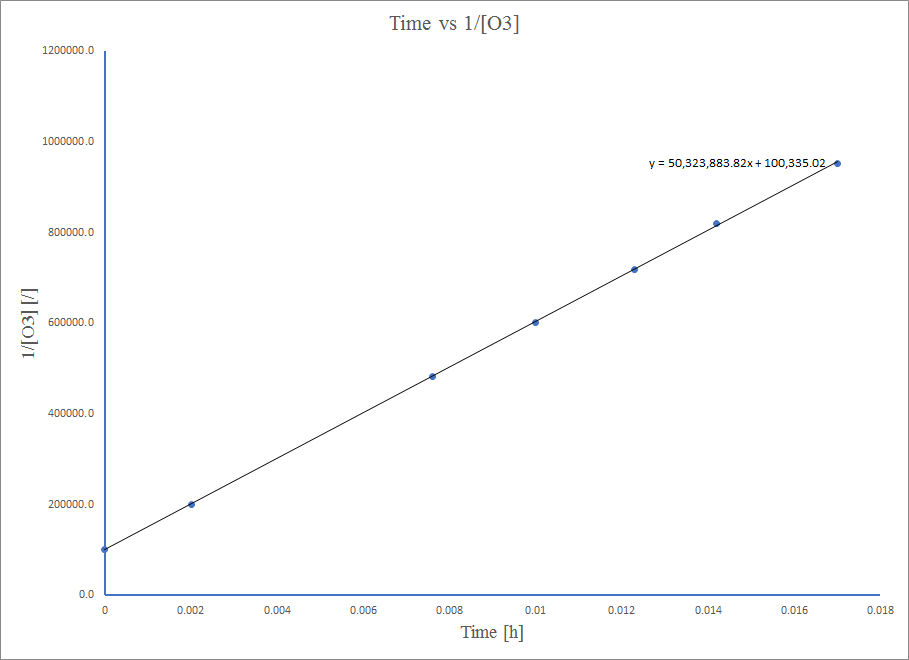
Mpango huo ni mstari mzuri, hivyo mmenyuko ni utaratibu wa pili.
Kwa equation ya pili ili,\( 1/[A] \ = k*t + 1/[A_0] \)
Hivyo, thamani ya K ni mteremko wa grafu Muda vs\( \frac{1}{\ce{O3}}\),
k = 50.3*10^6 L mol -1 h -1
- Jibu
-
Mpango huo ni mstari mzuri, hivyo mmenyuko ni utaratibu wa pili.
k = 50.1 L mol -1 h -1
Q12.4.5
Kutoka kwa data iliyotolewa, tumia njia ya kielelezo ili kuamua utaratibu na kiwango cha mara kwa mara cha majibu yafuatayo:
\[2X⟶Y+Z\]
| Muda (s) | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 |
|---|---|---|---|---|---|---|---|---|
| [X] (M) | 0.0990 | 0.0497 | 0.0332 | 0.0249 | 0.0200 | 0.0166 | 0.0143 | 0.0125 |
- Suluhisho
-
Ili kuamua utaratibu wa majibu tunahitaji kupanga njama kwa kutumia grafu tatu tofauti. Grafu zote tatu zitakuwa na muda kwa sekunde kama x-axis, lakini y-axis ni nini kitatofautiana. Grafu moja itapanga mkusanyiko dhidi ya wakati, pili itapanga logi ya asili ya ukolezi dhidi ya wakati, na nyingine itapanga 1/mkusanyiko dhidi ya nyakati. Kwa namna yoyote grafu matokeo katika mstari, tunajua kwamba lazima utaratibu wa majibu. Ikiwa tunapata mstari kwa kutumia grafu ya kwanza, itakuwa amri ya sifuri, ikiwa ni mstari wa grafu ya pili itakuwa amri ya kwanza, na ikiwa ni mstari wa grafu ya tatu itakuwa majibu ya pili. Sasa hebu tengeneze data ili kuamua utaratibu.



Tunaweza kuona wazi kwamba grafu ya tatu, ambayo viwanja 1/M dhidi ya wakati, ni mstari wa moja kwa moja wakati wengine wawili ni kidogo ikiwa. Kwa hiyo, tunaweza kuamua kwamba kiwango cha mmenyuko huu ni utaratibu wa pili. Hii pia inatuambia kwamba vitengo ya kiwango cha mara kwa mara ambayo inapaswa kuwa M -2 s -1 kwa majibu ya pili ili.
Kuamua kiwango cha mara kwa mara, kinachoitwa k, tunahitaji rahisi kutambua mteremko wa grafu ya tatu kwa kuwa hiyo ndiyo utaratibu wa mmenyuko huu. Ili kupata mteremko wa mstari, tunachukua pointi mbili na tuondoe maadili ya y na kisha tugawanye kwa tofauti ya maadili ya x. Hii ni jinsi ya kufanya hivyo:
Tumia pointi (5, 10.101) na (40, 80).
Sasa tumia haya ili kupata slop, aka kiwango cha mara kwa mara: (80-10.101)/(40-5) = 1.997 = k
Hivyo kiwango cha mara kwa mara kwa mmenyuko huu wa pili ni 1.997 M -1 s -1.
Q12.4.6
Je! Nusu ya nusu ya maisha ya kuoza kwa kwanza ya fosforasi-32? \(\ce{(^{32}_{15}P⟶^{32}_{16}S + e- )}\)Kiwango cha mara kwa mara kwa kuoza ni 4.85 × 10 -1 siku -1.
- Suluhisho
-
Hii ni majibu ya kwanza ili, hivyo tunaweza kutumia nusu maisha yetu equation chini:
\[t_{1/2}=\frac{0.693}{k}\nonumber \]
Kiwango cha mara kwa mara kinatupewa katika vitengo kwa siku. Wote tunapaswa kufanya, ni kuziba ndani ya equation.
\[t_{1/2}=\frac{0.693}{4.85*10^{-2}}\nonumber \]
\[=14.3\; days\nonumber \]A12.4.6
14.3 d
Q12.4.7
Je! Nusu ya nusu ya maisha ya kuoza kwa kwanza ya kaboni-14? \(\ce{(^6_{14}C⟶^7_{14}N + e- )}\)Kiwango cha mara kwa mara kwa kuoza ni 1.21 × 10 -4 mwaka -1.
- Suluhisho
-
Ili kupata maisha ya nusu, tunahitaji kutumia usawa wa nusu ya maisha ya kwanza. Athari zote za nusu za maisha hupata athari za kwanza.
Equation ya nusu ya maisha kwa utaratibu wa kwanza ni\[t_{1/2}=ln2/k \nonumber \] pamoja na k kuwa kiwango cha mara kwa mara. Kiwango cha mara kwa mara kwa kaboni-14 kilitolewa kama\(1.21 × 10^{-4} year^{−1}\).
Kuziba katika equation. \[t_{1/2}=ln2/(1.21 × 10^{−4} year^{−1})\nonumber \]na kutatua kwa\( t_{1/2}\).
Unapohesabu, maisha ya nusu ya kaboni-14 ni 5.73*10 3
- Jibu
-
Maisha ya nusu ya kaboni-14 imehesabiwa kuwa 5.73*10 3
Q12.4.8
Nusu ya nusu ya maisha ya kuharibika kwa NoCl wakati mkusanyiko wa NoCl ni 0.15 M? Kiwango cha mara kwa mara kwa mmenyuko huu wa pili ni 8.0 × 10 -8 L/mol/s.
- Suluhisho
-
Maisha ya nusu ya mmenyuko, t 1/2, ni kiasi cha muda kinachohitajika kwa mkusanyiko wa reactant kupungua kwa nusu ikilinganishwa na ukolezi wake wa awali. Wakati wa kutatua kwa nusu ya maisha ya mmenyuko, tunapaswa kwanza kuzingatia utaratibu wa majibu ili kuamua sheria ya kiwango cha. Katika kesi hii, tunaambiwa kuwa mmenyuko huu ni wa pili, kwa hiyo tunajua kwamba sheria ya kiwango cha jumuishi hutolewa kama:
\[\dfrac{1}{[A]} = kt + \dfrac{1}{[A]_0}\nonumber \]
Kutenganisha kwa muda, tunaona kwamba:
\[t_{1/2} = \dfrac{1}{k[A]_0}\nonumber \]
Sasa ni suala la kubadilisha maelezo tuliyopewa kuhesabu\(t_{1/2}\), ambapo kiwango cha mara kwa mara\({k}\), ni sawa na 8.0 × 10 -8 L/mol/s na mkusanyiko wa awali,\({[A]_0}\), ni sawa na 0.15 M:
\[t_{1/2} = \dfrac{1}{(8.0×10^{-8})(0.15)} = {8.33×10^7 seconds}\nonumber \]
- Jibu
-
8.33 × 10 7 s
Q12.4.9
Nusu ya nusu ya maisha ya kuharibika kwa O 3 wakati mkusanyiko wa O 3 ni 2.35 × 10 -6 M? Kiwango cha mara kwa mara kwa mmenyuko huu wa pili ni 50.4 L/mol/h.
- Suluhisho
-
Ongeza maandiko hapa. Usifute maandishi haya kwanza.
Tangu mmenyuko ni utaratibu wa pili, nusu yake ya maisha ni
\[t_{1/2}=\dfrac{1}{(50.4M^{-1}/h)[2.35×10^{-6}M]}\nonumber \]
Hivyo, nusu ya maisha ni masaa 8443.
Q12.4.10
Majibu ya kiwanja A kutoa misombo C na D ilionekana kuwa ya pili katika A. Kiwango cha mara kwa mara kwa mmenyuko kiliamua kuwa 2.42 L/mol/s Kama mkusanyiko wa awali ni 0.500 mol/L, ni thamani gani ya t 1/2?
- Suluhisho
-
Kama ilivyoelezwa katika swali, majibu ya kiwanja A yatasababisha kuundwa kwa misombo C na D. mmenyuko huu ulipatikana kuwa wa pili ili katika A. Kwa hiyo, tunapaswa kutumia equation ya pili ili kwa nusu ya maisha ambayo inahusiana kiwango cha mara kwa mara na viwango vya awali kwa nusu ya maisha:
\[t_{\frac{1}{2}}=\frac{1}{k[A]_{0}}\nonumber \]
Tangu tulipewa k (kiwango cha mara kwa mara) na mkusanyiko wa awali wa A, tuna kila kitu kinachohitajika kuhesabu maisha ya nusu ya A.
\[k=0.5\frac{\frac{L}{mol}}{s}\nonumber \]
\[[A]_{0}=2.42\frac{mol}{L}\nonumber \]
Wakati sisi kuziba katika taarifa kutokana taarifa kwamba vitengo kufuta nje kwa sekunde.
\[t_{\frac{1}{2}}=\frac{1}{\frac{2.42Lmol^{-}}{s}[0.500\frac{mol}{L}]}=0.826 s\nonumber \]
- Jibu
-
0.826 s
Q12.4.11
Maisha ya nusu ya mmenyuko wa kiwanja A kutoa misombo D na E ni dakika 8.50 wakati mkusanyiko wa awali wa A ni 0.150 mol/L. A au (b) utaratibu wa pili kwa heshima na A?
- Suluhisho
-
Panga vigezo vilivyopewa:
(nusu ya maisha ya A)\(t_{1/2}=8.50min\)
(mkusanyiko wa awali wa A)\([A]_{0}=0.150mol/L\)
(lengo la A)\([A]=0.0300mol/L\)Kupata kiwango cha mara kwa mara k, kwa kutumia nusu ya maisha formula kwa kila utaratibu husika. Baada ya kupata k, tumia sheria ya kiwango cha jumuishi husika na kila utaratibu na viwango vya awali na vya lengo la A ili kupata muda ulichukua kwa mkusanyiko kushuka.
(a) utaratibu wa kwanza kwa heshima na A
(nusu ya maisha)\(t_{1/2}=\frac{ln(2)}{k}=\frac{0.693}{k}\)
(upya kwa k)\(k=\frac{0.693}{t_{1/2}}\)
(kuziba katika t 1/2 = 8.50 min)\(k=\frac{0.693}{8.50min}=0.0815min^{-1}\)(jumuishi kiwango cha sheria)\(ln[A]=-kt+ln[A]_{0}\)
(upya kwa t)\(ln(\frac{[A]}{[A]_{0}})=-kt\)
\(-ln(\frac{[A]}{[A]_{0}})=kt\)
\(ln(\frac{[A]}{[A]_{0}})^{-1}=kt\)
\(ln(\frac{[A]_{0}}{[A]})=kt\)
\(t=\frac{ln(\frac{[A]_{0}}{[A]})}{k}\)
(kuziba katika vigezo)\(t=\frac{ln(\frac{0.150mol/L}{0.0300mol/L})}{0.0815min^{-1}}=\frac{ln(5.00)}{0.0815min^{-1}}=19.7min\)(b) utaratibu wa pili kwa heshima na A
(nusu ya maisha)\(t_{1/2}=\frac{1}{k[A]_{0}}\)
(upya kwa k)\(k=\frac{1}{t_{1/2}[A]_{0}}\)
(kuziba katika vigezo)\(k=\frac{1}{(8.50min)(0.150mol/L)}=\frac{1}{1.275min\cdot mol/L}=0.784L/mol\cdot min\)(jumuishi kiwango cha sheria)\(\frac{1}{[A]}=kt+\frac{1}{[A]_{0}}\)
(upya kwa t)\(\frac{1}{[A]}-\frac{1}{[A]_{0}}=kt\)
\(t=\frac{1}{k}(\frac{1}{[A]}-\frac{1}{[A]_{0}})\)
(kuziba katika vigezo)\(t=\frac{1}{0.784L/mol\cdot min}(\frac{1}{0.0300mol/L}-\frac{1}{0.150mol/L})=\frac{1}{0.784L/mol\cdot min}(\frac{80}{3}L/mol)=34.0min\)
- Jibu
-
a) 19.7 min
b) 34.0 min
Q12.4.12
Baadhi ya bakteria ni sugu kwa penicillin antibiotiki kwa sababu huzalisha penicillinase, enzyme yenye uzito wa masi ya 3 × 10 4 g/mol inayobadilisha penicillin kuwa molekuli zisizo na kazi. Ingawa kinetiki ya athari za kichocheo cha enzyme zinaweza kuwa ngumu, kwa viwango vya chini mmenyuko huu unaweza kuelezewa na equation ya kiwango ambacho ni amri ya kwanza katika kichocheo (penicillinase) na ambayo pia inahusisha mkusanyiko wa penicillin. Kutoka data zifuatazo: 1.0 L ya suluhisho iliyo na 0.15 μg (0.15 × 10 -6 g) ya penicillinase, kuamua utaratibu wa mmenyuko kuhusiana na penicillin na thamani ya kiwango cha mara kwa mara.
| [Penicillin] (M) | Kiwango (mol/L/min) |
|---|---|
| 2.0 × 10 -6 | 1.0 × 10 -10 |
| 3.0 × 10 -6 | 1.5 × 10 -10 |
| 4.0 × 10 -6 | 2.0 × 10 -10 |
- Suluhisho
-
Hatua ya kwanza ni kutatua kwa utaratibu au majibu. Hii inaweza kufanyika kwa kuanzisha maneno mawili ambayo yanalinganisha kiwango cha mara kwa mara mara mkusanyiko wa molar wa penicillin uliofufuliwa kwa nguvu ya utaratibu wake. Mara baada ya kuwa na maneno yote kuanzisha, tunaweza kugawanya yao kufuta k (kiwango cha mara kwa mara) na kutumia logarithm msingi kutatua kwa exponent, ambayo ni ili. Itaonekana kama hii.
kiwango (mol/L/min) =k [M] x
(1.0 x 10 -10) =k [2.0 x 10 -6] x
(1.5 x 10 -10) =k [3.0 x 10 -6] x
Kugawanya equations mbili matokeo katika kujieleza:
(2/3) = (2/3) x
*Equation moja ya uwiano pia inaweza kuanzishwa ili kutatua kwa utaratibu wa majibu:
*\[\frac{rate_{1}}{rate_{2}}=\frac{k[Penicillin]_{1}^{x}}{k[Penicillin]_{2}^{x}}\nonumber \]
*Sisi kisha kutatua kwa x kwa mtindo sawa.
*\[\frac{1.0x10^{-10}}{1.5x10^{-10}}=\frac{[2.0x10^{-6}]^{x}}{[3.0x10^{-6}]^{x}}\nonumber \]
Sasa tunaweza kutumia logarithm asili kutatua kwa x, au tu na intuitively kuona kwamba ili equation kufanya kazi, x lazima kuwa sawa na moja. Hivyo, majibu ni ya kwanza.
Sasa kwa kuwa tuna utaratibu wa majibu, tunaweza kuendelea kutatua kwa thamani ya kiwango cha mara kwa mara. Kubadilisha x=1 katika equation yetu ya kwanza hutoa usemi:(1 x 10 -10) =k [2.0 x 10 -6] 1
k= (1 x 10 -10)/(2 x 10 -6)
k= (5 x 10 -5) min -1
Tuna kitengo cha min -1 kwa sababu tumegawanyika (mol/L/min) kwa molarity, ambayo iko katika (mol/L), kutoa kitengo cha min -1.
Tulipewa vipande viwili muhimu vya habari ili kumaliza tatizo. Inasemekana kuwa enzyme ina uzito wa Masi ya 3 × 10 4 g/mol, na kwamba tuna suluhisho la lita moja ambalo lina (0.15 x 10 -6 g) ya penicillinase. Kugawanya kiasi cha gramu kwa uzito wa Masi huzaa 5 x 10 -12 moles.
(0.15 x 10 -6) g/(3 x 10 4) g/mol = (5 x 10 -12) mol
Sasa kwa kuwa tuna kiasi cha moles, tunaweza kugawanya kiwango cha mara kwa mara kwa thamani hii.
(5 x 10 -5) min -1/(5 x 10 -12) mol = (1 x 10 7) mol -1 min -1
Jibu
-
Mmenyuko ni utaratibu wa kwanza na k = 1.0 × 10 7 mol -1 min -1
Q12.4.13
Wote technetium-99 na thallium-201 hutumiwa kutengeneza misuli ya moyo kwa wagonjwa walio na matatizo ya moyo ya watuhumiwa. Nusu ya maisha ni 6 h na 73 h, kwa mtiririko huo. Ni asilimia gani ya mionzi itabaki kwa kila isotopu baada ya siku 2 (48 h)?
- Suluhisho
-
Tatizo hili linatuuliza asilimia ya mionzi iliyobaki baada ya muda fulani kwa isotopu zote mbili baada ya masaa 48. Tunapaswa kutambua equation ambayo itatusaidia kutatua hili na tunaweza kuamua kwamba tunaweza kuamua habari hii kwa kutumia equation ya kwanza ili.
Hii equation Ln (N/N o) = -kt anasema kwamba logi Asili ya sehemu iliyobaki ni sawa na kiwango mara kwa mara mara wakati. Kuamua kiwango cha mara kwa mara, tunaweza pia kukokotoa .693 juu ya nusu ya maisha iliyotolewa katika habari.
Kwa Technetium-99 tunaweza kuamua kiwango cha mara kwa mara kwa kuziba kwenye equation ya pili: .693/6 hrs= .1155 h -1
Sasa kwa kuwa tuna kiwango cha mara kwa mara tunaweza kuziba: Ln (N/N o) =- (.1155h -1) (48h) hivyo Ln (N/N o) =-5.544 na ikiwa tunachukua kinyume cha logi ya asili, tunapata (N/N o) =3.9x10 -3 na ikiwa tunazidisha hii kwa 100, tunapata .39% iliyobaki.
Tunaweza kufanya mchakato huu huo kwa Thallium-201 na Plugin: .693/73 hrs= .009493151 h -1 na wakati sisi kuziba hii katika kwanza ili equation sisi kupata:
Ln (N/N o) =- (.009493h -1) (48h) hivyo Ln (N/N o) =-.45567248 na tunapopata kinyume cha logi ya asili, tunapata (N/N o) =.6340 na tunapoongezeka kwa 100, tunapata 63.40% iliyobaki ambayo ina maana tangu nusu yake ya maisha ni masaa 73 na masaa 48 tu yamepita, nusu ya kiasi bado zinazotumiwa.
- Jibu
-
Technetium-99:0.39%
Thallium-201:63.40%
Q12.4.14
Kuna molekuli mbili na formula C 3 H 6. Propene\(\ce{CH_3CH=CH_2}\), ni monoma ya polymer polypropen, ambayo hutumiwa kwa mazulia ya ndani ya nje. Cyclopropane hutumiwa kama anesthetic:

Wakati joto hadi 499 °C, cyclopropane rearranges (isomerizes) na huunda propene yenye mara kwa mara ya kiwango cha 5.95 × 10 -4 s -1. Nusu ya maisha ya mmenyuko huu ni nini? Ni sehemu gani ya cyclopropane iliyobaki baada ya 0.75 h saa 499 °C?
- Suluhisho
-
Tumia equation\[ t{_1}{_/}{_2} = \frac{ln2} k\nonumber \] kwani hii ni majibu ya kwanza. Unaweza kusema kwamba hii ni majibu ya kwanza kutokana na vitengo vya kipimo cha kiwango cha mara kwa mara, ambayo ni s -1. Maagizo tofauti ya athari husababisha viwango tofauti vya kiwango, na kiwango cha mara kwa mara cha s -1 kitakuwa cha kwanza.
Plug katika equation, na kupata nusu maisha = 1164.95 sekunde. Ili kubadilisha hii kwa masaa, tungegawanya nambari hii kwa sekunde 300/saa, ili kupata masaa 0.324.
Matumizi jumuishi amri ya kwanza ya kiwango cha sheria\[ln\frac{[A]}{[A]_0} = -kt\nonumber \]. Katika equation hii, [A] 0 inawakilisha kiasi cha awali cha kiwanja kilichopo wakati 0, wakati [A] inawakilisha kiasi cha kiwanja kinachoachwa baada ya mmenyuko imetokea. Kwa hiyo, sehemu hiyo\[\frac{[A]}{[A]_0}\nonumber \] ni sawa na sehemu ya cyclopropane iliyobaki baada ya muda fulani, katika kesi hii, masaa 0.75.
Mbadala x kwa sehemu ya\[\frac{[A]}{[A]_0}\nonumber \] ndani ya sheria jumuishi kiwango:\[ln\frac{[A]}{[A]_0} = -kt\nonumber \]\[ln(x) = -5.95x10^{-4}(0.75)\nonumber \]\[x=e^{(-0.000595)(0.75)}\nonumber \] = 0.20058 = 20%.
Kwa hiyo, maisha ya nusu ni masaa 0.324, na asilimia 20 ya cyclopropane itabaki kama 80% itaunda propene.
- Jibu
-
Masaa 0.324; 20% inabakia
Q12.4.16
Fluorine-18 ni isotopu ya mionzi inayooza kwa chafu ya positroni ili kuunda oksijeni-18 yenye nusu ya maisha ya dakika 109.7. (Positron ni chembe yenye wingi wa elektroni na kitengo kimoja cha chaji chanya; equation nyuklia ni\(\ce{^{18}_9F ⟶ _8^{18}O + ^0_{1}e^+}\).) Waganga hutumia 18 F kujifunza ubongo kwa kuingiza kiasi cha glucose iliyobadilishwa na fluoro-ndani ya damu ya mgonjwa. Glucose hukusanya katika mikoa ambapo ubongo ni kazi na inahitaji chakula.
- Ni kiwango gani cha mara kwa mara kwa ajili ya kuharibika kwa fluorine-18?
- Ikiwa sampuli ya glucose iliyo na fluorini ya mionzi 18 inakabiliwa ndani ya damu, ni asilimia gani ya radioactivity itabaki baada ya 5.59 h?
- Inachukua muda gani kwa 99.99% ya 18 F kuoza?
- Suluhisho
-
a) Uozo wa nyuklia wa isotopu ya kipengele unawakilishwa na equation ya kwanza:
ln (N/N0) = -kt
Ambapo t ni wakati, N0 ni kiasi cha awali cha dutu hii, N ni kiasi cha dutu baada ya muda t, na k ni kiwango cha mara kwa mara. Tunaweza kupanga upya equation na kujitenga k ili tuweze kutatua kwa kiwango cha mara kwa mara:
k = [-ln (N/N0)]/t
Tunapewa kwamba fluorine-18 ina nusu ya maisha ya dakika 109.7. Kwa kuwa tuna nusu ya maisha, tunaweza kuchagua thamani ya kiholela kwa N 0 na kutumia nusu ya thamani hiyo kwa N. katika kesi hii, tunachagua 100 kwa N 0 na 50 kwa N Sasa tunaweza kuziba maadili hayo katika equation hapo juu na kutatua kwa k.
k = [-ln (50/100)]/109.7
k = 0.6931/109.7 = 0.006319 min -1
Kiwango cha mara kwa mara kwa mmenyuko huu ni 0.006319 min -1.
b) Kwa tatizo hili, tunaweza kutumia equation sawa kutoka sehemu a:
ln (N/N0) = -kt
Hata hivyo, wakati huu tunapewa kiasi cha muda uliopita badala ya nusu ya maisha, na tunaulizwa kuamua asilimia ya radioactivity ya florini-18 iliyobaki baada ya wakati huo. Katika tatizo hili, tunapaswa kuziba maadili kwa N0, k (kuamua kutoka sehemu a), na t.
Lakini kwanza, kwa kuwa tunapewa muda uliopita kwa masaa, lazima tuibadilishe kuwa dakika:
Masaa 5.59 x (dakika 60/ masaa 1) = dakika 335.4
Hii inatupa thamani ya t Pia tuna maadili kwa k (0.006319 min -1) na N 0 (tena idadi ya kiholela.) Sasa tunaweza kuziba maadili katika equation ya awali, kutupa:
ln (N/100) = - (0.006319) (335.4)
Sisi kutatua equation hii kwa kuchukua kielelezo cha pande zote mbili:
e ln (N/100) = e - (0.006319) (335.4)
ambapo e ln sawa 1 na sasa tunaweza tu kutatua kwa N:
N/100 = e - (0.006319) (335.4)
N = [e - (0.006319) (335.4)] x 100 = 12.0
Kwa kuwa 100 ilitumiwa kama kiasi cha awali na 12.0 iliamua kama kiasi kilichobaki, 12.0 inaweza kutumika kama asilimia ya kiasi kilichobaki cha radioactivity ya fluorine-18. Hivyo asilimia ya radioactivity ya florine-18 iliyobaki baada ya masaa 5.59 ni 12.0%.
c) Sehemu hii ya swali ni sawa na sehemu mbili zilizopita, lakini wakati huu tunapewa kiasi cha awali cha mionzi, kiasi cha mwisho cha mionzi na tunaulizwa kuamua muda gani ilichukua kwa kiasi hicho cha mionzi kuoza. Tunaweza kutumia equation sawa:
ln (N/N0) = -kt
Hata hivyo, sasa tunapewa N na N 0 na tumeamua k kutoka kabla. Tunaambiwa kuwa 99.99% ya radioactivity imeharibika, hivyo tunaweza kutumia 100 na 0.01 kwa N 0 na N kwa mtiririko huo. Sisi kuziba maadili haya katika equation, kutatua kwa t, na kupata
ln (0.01/1000) = -0.006319t
-9.21 = -0.006319t
t = dakika 1458
Kwa hiyo, inachukua dakika 1458 kwa 99.99% ya radioactivity kuoza.
- Jibu
-
a) 0.006319 min -1
b) 12.0%
c) dakika 1458
Q12.4.17
Tuseme kwamba nusu ya maisha ya steroids zilizochukuliwa na mwanariadha ni siku 42. Kutokana kwamba steroids biodegrade na mchakato wa kwanza ili, muda gani itachukua kwa ajili\(\dfrac{1}{64}\) ya kipimo cha awali kubaki katika mwili mwanamichezo?
- Suluhisho
-
siku 252
kwa majibu ya kwanza: t 1/2 = 0.693/k
k = 0.693/42
k = 0.0165
kwa majibu ya kwanza ili: [A] = [A] 0 e -kt
1/64 awali ina maana kwamba: [A] = 1/64 [A] 0
Kwa hiyo: 1/64 [A] 0 = [A] 0 e -0.0165t
t = siku 252
Q12.4.18
Hivi karibuni, mifupa ya Mfalme Richard III ilipatikana chini ya kura ya maegesho nchini Uingereza. Ikiwa sampuli za tishu kutoka mifupa zina karibu 93.79% ya kaboni-14 inavyotarajiwa katika tishu zilizo hai, Mfalme Richard III alikufa mwaka gani? Maisha ya nusu ya kaboni-14 ni miaka 5730.
- Suluhisho
-
Ili kujua ni mwaka gani Mfalme Richard III alikufa, kuweka [A]/[A 0] (asilimia ya kaboni-14 bado zilizomo) sawa na 0.5 muda (t) /nusu maisha (t 1/2) au kutumia equation N (t) = N 0 e -rt.
Kutumia equation ya kwanza:
\(A/A_{0}\)=\(0.5^{t/t_{1/2}}\) kuziba katika idadi fulani\(.9379 = 0.5^{t/5730}\) na kutatua kwa t.
\(ln.9379\)=\((t/5730)(ln0.5)\) (kutumia utawala wa magogo)
\(-.0641\)=\((t/5730)(-.693)\)
\(-367.36\)=\(-.693t\)
\(t = 530.1 years\)
Kutumia tatizo\(N(t) = N_{0}e^{-rt}\) hili ni kutatuliwa na yafuatayo:
\(1/2 = e^{-5730r}\)
\(r = 0.000121\)
Sasa kwa kuwa tunajua nini r ni, tunaweza kutumia thamani hii katika formula yetu ya awali na kutatua kwa t, kiasi cha miaka ambayo yamepita.
Wakati huu, tunatumia 93.78, asilimia ya kaboni-14 iliyobaki kama N (t) na 100 kama ya awali, N 0.
\(93.78 = 100e^{-0.000121t}\)
\(t = 530.7\)miaka
Njia nyingine ya kufanya hivyo ni kwa kutumia equations hizi mbili:
λ =\(\dfrac{0.693}{t_{1/2}}\) na\(\dfrac{n_{t}}{n_{0}}\) = -λt
\(n_{t}\)= mkusanyiko wakati t (93.79)
\(n_{0}\)= mkusanyiko wa awali (100)
Kwanza kutatua kwa lambda au mara kwa mara ya kuoza kwa kuziba katika maisha ya nusu.
Kisha kuziba lambda na namba nyingine katika equation ya pili, na kutatua kwa t- ambayo inapaswa kuwa sawa na miaka 530.1 pia.
Ikiwa tunataka kujua mwaka gani Mfalme Richard III alikufa, tunachukua mwaka huu, 2017, na tuondoe miaka 530. Kufanya hivyo, tunaona kwamba Mfalme Richard III alikufa mwaka 1487.
- Jibu
-
Mfalme Richard III alikufa mwaka 1487
Q12.4.19
Nitroglycerine ni kulipuka sana nyeti. Katika mfululizo wa majaribio yaliyodhibitiwa kwa makini, sampuli za mlipuko zilichomwa moto hadi 160 °C na utengano wao wa kwanza ulijifunza. Tambua kiwango cha wastani cha kiwango cha kila jaribio kwa kutumia data zifuatazo:
| Awali [C 3 H 5 N 3 O 9] (M) | 4.88 | 3.52 | 2.29 | 1.81 | 5.33 | 4.05 | 2.95 | 1.72 |
|---|---|---|---|---|---|---|---|---|
| (s) | 300 | 300 | 300 | 300 | 180 | 180 | 180 | 180 |
| % Imeharibika | 52.0 | 52.9 | 53.2 | 53.9 | 34.6 | 35.9 | 36.0 | 35.4 |
- Suluhisho
-
Kwanza tunahitaji kuelewa swali linaloomba: kiwango cha wastani cha mara kwa mara. Kiwango cha wastani cha mara kwa mara ni variable “k” wakati wa kujadili kinetiki na inaweza kuelezwa kama mara kwa mara uwiano katika equation inayoonyesha uhusiano kati ya kiwango cha mmenyuko wa kemikali na viwango vya vitu vinavyoitikia. Tukijua kwamba tunahitaji kupata K katika mmenyuko huu wa kwanza, tunaweza kuangalia fomu ambazo ni pamoja na “k,” viwango vya awali na vya mwisho\([A]_o and [A]_t\), na muda wa nusu ya maisha “t.” Kwa kuwa hii ni ya kwanza ili majibu, tunaweza kuangalia equations kwanza ili, na kufanya hivyo sisi kupata moja kuwa ni pamoja na vigezo aliyopewa katika swali:\[\ln[A]_t=-kt+\ln[A]_o\nonumber \]
Kwa majibu ya kwanza, tuna mkusanyiko wa awali wa 4.88 M, na asilimia imeharibika. Ili kupata mkusanyiko wa mwisho, tunapaswa kuzidisha mkusanyiko wa awali kwa asilimia iliyoharibika ili kujua kiasi gani kilichoharibika, na kuondoa hiyo kutoka kwa asili ili kujua ni kiasi gani kinachoachwa: 4.88M x 0.52= 2.54 M na 4.88M-2.54M=2.34M
Sasa, tuna vigezo tunahitaji, na sisi kuziba ndani equation hapo juu:
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[2.34M]=-k(300s)+\ln[4.88M]\)
k=\({-(\ln[2.34M]-\ln[4.88M])}\over 300\)
\(k=2.45x10^{-3}\)
Kwa kuwa anauliza kiwango cha mara kwa mara ya kila jaribio, sasa tunapaswa kufanya utaratibu huo kwa kila kuweka data ili kupata kiwango cha mara kwa mara:
Jaribio la pili
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[1.66M]=-k(300s)+\ln[3.52M]\)
k=\({-(\ln[1.66M]-\ln[3.52M])}\over 300\)
\(k=2.51x10^{-3}\)
Jaribio la tatu
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[1.07M]=-k(300s)+\ln[2.29M]\)
k=\({-(\ln[1.07M]-\ln[2.29M])}\over 300\)
\(k=2.54x10^{-3}\)
Jaribio la nne
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[0.834M]=-k(300s)+\ln[1.81M]\)
k=\({-(\ln[0.834M]-\ln[1.81M])}\over 300\)
\(k=2.58x10^{-3}\)
Tano majaribio
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[3.49M]=-k(180s)+\ln[5.33M]\)
k=\({-(\ln[3.49M]-\ln[5.33M])}\over 180\)
\(k=2.35x10^{-3}\)
Jaribio la sita
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[2.60M]=-k(180s)+\ln[4.05M]\)
k=\({-(\ln[2.60M]-\ln[4.05M])}\over 180\)
\(k=2.46x10^{-3}\)
Jaribio la Saba
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[1.89M]=-k(180s)+\ln[2.95M]\)
k=\({-(\ln[1.89M]-\ln[2.95M])}\over 180\)
\(k=2.47x10^{-3}\)
Jaribio la nane
\(\ln[A]_t=-kt+\ln[A]_o\)
\(\ln[1.11M]=-k(180s)+\ln[1.72M]\)
k=\({-(\ln[1.11M]-\ln[1.72M])}\over 180\)
\(k=2.43x10^{-3}\)
- Jibu
-
[A] (M) k × 10 (s -1) 4.88 2.45 3.52 2.51 2.29 2.54 1.81 2.58 5.33 2.35 4.05 2.44 2.95 2.47 1.72 2.43
Q12.4.20
Kwa kipindi cha miaka 10, hidrocarbon isokefu 1,3-butadiene\(\ce{(CH2=CH–CH=CH2)}\) imeweka nafasi ya 38 kati ya kemikali za juu za viwanda vya 50. Inatumiwa hasa kwa ajili ya utengenezaji wa mpira wa synthetic. Isoma ipo pia kama cyclobutene:

Isomerization ya cyclobutene kwa butadiene ni ya kwanza na mara kwa mara ya kiwango kimepimwa kama 2.0 × 10 —4 s -1 saa 150 °C katika chupa 0.53-L. Kuamua shinikizo la sehemu ya cyclobutene na ukolezi wake baada ya dakika 30.0 ikiwa mmenyuko wa isomerization unafanywa saa 150 °C na shinikizo la awali la 55 torr.
- Suluhisho
-
Kwa kuwa hii ni ya kwanza ili majibu, jumuishi kiwango cha sheria ni:\([A_{t}]=[A_{0}]e^{-kt}\)
Sehemu ya shinikizo: Matumizi jumuishi kiwango cha sheria kupata shinikizo sehemu katika 30 dakika:Matumizi\(A_0\) = 55 torr, t = 30 min, na k =\(2.0 * 10^{-4}s^{-1}\) kutatua jumuishi kiwango cha sheria equation:
\([A_{30}]=(55 torr)*e^{-(2.0x10^{-4}\frac{1}{sec})(30min\cdot\frac{60sec}{1 min})}\)
Tatua equation hii kupata:
\([A_{30}]=(55 torr)*e^{-0.36}\)
\(A_{30}]\)= 38.37 torr.
Mkazo wa awali: Pata mkusanyiko wa awali ukitumia sheria bora ya gesi.
Sheria bora ya gesi hutolewa na\(PV = nRT → n = \frac{PV}{RT}\). Tumia fomu hii ya sheria ya gesi kutatua kwa mkusanyiko wa awali n.
Tumia V = 0.53L, R = 0.08206\(\frac{L*atm}{mol*L}\), T = 423.15 K, na P\(\frac{1 atm}{760}\) = 0.07237 atm.
Kutatua bora gesi equation kutumia maadili haya:
\(n=\frac{(55torr)(0.53L)}{(0.08206\frac{L*atm}{mol*K})(423.15K)} = 0.00110\)moles cyclobutene.
Sasa tafuta mkusanyiko wa awali wa cyclobutene\(A_0\) kwa kutumia equation\([A_0] = \frac{n}{V}\):
\(A_0 = \frac{n}{V} = \frac{0.00110 moles}{0.53 L} = 0.00208 M\)
Mkazo katika dakika 30: Pata mkusanyiko wa cyclobutene kwa dakika 30 kwa kutumia sheria ya kiwango cha jumuishi iliyotolewa hapo juu, kwa kutumia muda t = dakika 30, au sekunde 1800.
\([A_{30}]=(0.00208M)e^{-0.36}= 0.00145M\)
Hivyo kwa dakika 30, mkusanyiko wa cyclobutene ni 0.00145 M, na shinikizo la sehemu ni 38.37 torr.
- Jibu
-
Sehemu ya shinikizo: 38.37 torr.
Mkazo: 0.00145 M
12.5: Nadharia ya mgongano
Q12.5.1
Athari za kemikali hutokea wakati majibu yanapogongana. Ni mambo mawili ambayo yanaweza kuzuia mgongano kutoka kuzalisha mmenyuko wa kemikali?
- Suluhisho
-
Sababu mbili ambazo zinaweza kuzuia mgongano kutokana na kuzalisha mmenyuko wa kemikali ni:
1. Nishati ya kinetic ya molekuli
Ili athari za kemikali kutokea, molekuli zinahitaji kasi ya kutosha kushinda nishati ya uanzishaji wa chini inayohitajika kuvunja vifungo vya zamani na kuunda vifungo vipya na molekuli nyingine. Katika joto la juu, molekuli zina kiwango cha chini cha nishati ya kinetic inayohitajika ambayo inahakikisha migongano itakuwa na nguvu ya kutosha kusababisha mmenyuko.
2. Ya mwelekeo wa molekuli wakati wa mgongano
Molekuli mbili zinapaswa kugongana katika mwelekeo sahihi ili majibu yatoke. Molekuli zinapaswa kuelekea vizuri kwa molekuli nyingine ili kugongana kwenye hali ya uanzishaji sahihi.
Q12.5.2
Wakati kila mgongano kati ya majibu husababisha mmenyuko, ni nini kinachoamua kiwango ambacho mmenyuko hutokea?
- Suluhisho
-
Inapaswa kuwa na mawasiliano kati ya majibu kwa mmenyuko kutokea. Zaidi ya reactants hugongana, mara nyingi athari zinaweza kutokea. Mambo ambayo huamua viwango vya majibu ni pamoja na mkusanyiko wa reactants, joto, majimbo ya kimwili ya reactants, eneo la uso, na matumizi ya kichocheo. Kiwango cha majibu huongezeka kwa kawaida kama mkusanyiko wa ongezeko la reactant. Kuongezeka kwa joto huongeza wastani wa nishati ya kinetic ya molekuli, na kusababisha wao kugongana mara nyingi zaidi, ambayo huongeza kiwango cha majibu. Wakati reactants mbili ziko katika awamu moja ya maji, chembe zao hugongana mara kwa mara, ambayo huongeza kiwango cha majibu. Ikiwa eneo la uso wa reactant linaongezeka, chembe zaidi zinaonekana kwa reactant nyingine kwa hiyo migongano zaidi hutokea na kiwango cha mmenyuko kinaongezeka. Kichocheo kinashiriki katika mmenyuko wa kemikali na huongeza kiwango cha majibu bila kubadilisha yenyewe.
Q12.5.3
Nishati ya uanzishaji wa mmenyuko ni nini, na nishati hii inahusianaje na tata iliyoamilishwa ya mmenyuko?
- Suluhisho
-

Nishati ya uanzishaji ni kizuizi cha nishati ambacho kinapaswa kushinda ili majibu yatoke. Ili kupata molekuli katika hali ambayo inawawezesha kuvunja na kuunda vifungo, molekuli lazima ziingizwe (kuharibika, au kuinama) katika hali isiyo imara inayoitwa hali ya mpito. Hali ya mpito ni hali ya juu-nishati, na kiasi fulani cha nishati — nishati ya uanzishaji — lazima iongezwe ili molekuli ifikie. Kwa sababu hali ya mpito ni imara, molekuli reactant wala kukaa huko kwa muda mrefu, lakini haraka kuendelea na hatua ya pili ya mmenyuko kemikali. Tata ulioamilishwa ni nishati ya juu ya hali ya mpito ya majibu.
Q12.5.5
Eleza jinsi mbinu za kielelezo zinaweza kutumiwa kuamua nishati ya uanzishaji wa mmenyuko kutoka kwa mfululizo wa data ambayo inajumuisha kiwango cha majibu kwa joto tofauti.
- Suluhisho
-
Njia hii inategemea equation ya Arrhenius ambayo inaweza kutumika kuonyesha athari za mabadiliko ya joto kwenye kiwango cha mara kwa mara, na kwa hiyo juu ya kiwango cha mmenyuko.
Mara kwa mara kiwango ni tofauti na panya ya mmenyuko kwa kuwa kiwango cha mmenyuko ni kipimo cha jinsi kasi au polepole mmenyuko wa kemikali unafanyika ilhali kiwango cha mara kwa mara ni mara kwa mara kinachoonyesha uhusiano kati ya kiwango cha mmenyuko na viwango vya reactants au bidhaa.
Kwa mfano, kwa majibu\(A + B \rightarrow C\), the rate law would be:
\(rate = k[A]^a[B]^b\)
k = kiwango cha mara kwa mara
[A] = mkusanyiko wa reactant A
a = utaratibu wa majibu kwa heshima na A
[B] = mkusanyiko wa reactant B
b = utaratibu wa majibu kwa heshima na B
Hata hivyo, kiwango cha mara kwa mara kinaendelea mara kwa mara tu ikiwa unabadilisha mkusanyiko wa wahusika. Ikiwa unabadilisha joto au kichocheo cha mmenyuko, kiwango cha mara kwa mara kitabadilika na hii inaonyeshwa na equation ya Arrhenius:
\(k = Ae^\frac{-E_a}{RT}\)
\(ln \left(\frac{k_1}{k_2}\right) = \left(\frac{-E_a}{R}\right)\left(\frac{1}{T_1} - \frac{1}{T_2}\right)\)
k = kiwango cha mara kwa mara
A = sababu ya mzunguko
\(E_a\) = activation energy
e = kazi ya kielelezo,\(e^x\)
R = gesi mara kwa mara
T = joto (K)

Kwa maneno mengine, nishati ya uanzishaji wa mmenyuko,\(E_a\), from a series of data that includes the rate of reaction, k, at varying temperatures can be determined by graphing it on a plot of \(\ln k\) versus \(\frac{1}{T}\). You can then use the slope of the graph you have plotted to solve for \(E_a\) by setting the slope equal to \(\frac{-E_a}{R}\).
Q12.5.6
How does an increase in temperature affect rate of reaction? Explain this effect in terms of the collision theory of the reaction rate.
S12.5.6
Collision theory states that the rates of chemical reactions depend on the fraction of molecules with the correct orientation, fraction of collisions with required energy, and the collision frequency. Because the fraction of collisions with required energy is a function of temperature, as temperature increases, the fraction of collisions with required energy also increases. The kinetic energy of reactants also increases with temperature which means molecules will collide more often increasing collisions frequency. With increased fraction of collisions with required energy and collisions frequency, the rate of chemical reaction increases. We see mathematically, from the Arrhenius equation, that temperature and the rate constant are related.
\[k=Ae^{\frac {E_a}{RT}}\]
where k is the rate constant, A is a specific constant, R is 8.3145 J/K, Ea is the reaction-specific activation energy in J, and T is temperature in K. We see from the equation that k is very sensitive to changes in the temperature.
Q12.5.7
The rate of a certain reaction doubles for every 10 °C rise in temperature.
- How much faster does the reaction proceed at 45 °C than at 25 °C?
- How much faster does the reaction proceed at 95 °C than at 25 °C?
- Solution
-
By finding the difference in temperature, 45 °C - 25 °C, we get 20 °C. Since the rate of the reaction doubles every 10 °C increase in temperature and the rate of the reaction experienced a 20 °C increase in temperature, we see that the reaction rate doubled twice (22 = 4). As a result, the reaction proceeds four times faster.
Following the same process as in part a, we get the difference in temperature to be 70 °C. Since the rate of the reaction doubles every 10 °C increase in temperature and the system experienced a 70 °C change, we see that the reaction doubled seven times (27 = 128). We can see the reaction proceeds 128 times faster.
(a) 4-times faster (b) 128-times faster
Q12.5.8
In an experiment, a sample of NaClO3 was 90% decomposed in 48 min. Approximately how long would this decomposition have taken if the sample had been heated 20 °C higher?
S12.5.8
First off, it is important to recognize that this decomposition reaction is a first-order reaction, which can be written as follows: \(\mathrm2NaClO_3\to2NaCl + 3O_2\)
Understanding this, it is important to be able to then be able to recognize which equation would be most useful given the initial conditions presented by the question. Since we are dealing with time, percentage of material left, and temperature, the only viable equation that could relate all of this would be the Arrhenius Equation, which is written as follows: \(\mathrm \ln(\frac{k_2}{k_1}) = \frac {Ea}{R}({\frac1{t_1}}-{\frac{1}{t_{2}}})\)
However, this problem does not give us enough information such as what the activation energy is or the initial temperature in order to mathematically solve this problem. Additionally, the problem tells us to approximate how long the decomposition would take, which means we are asked to answer this question conceptually based on our knowledge of thermodynamics and reaction rates. As a general rule of thumb, we know that for every 10˚C rise in temperature the rate of reaction doubles. Since the question tells us that there is a 20˚C rise in temperature we can deduce that the reaction rate doubles twice, as per the general rule mentioned before. This means the overall reaction rate for this decomposition would quadruple, or would be 4 times faster than the reaction rate at the initial temperature.
We can gut check this answer by recalling how an increase in the average kinetic energy (temperature) decreases the time it takes for the reaction to take place and increase the reaction rate. Thus, if we increase the temperature we should have a faster reaction rate.
Q12.5.9
The rate constant at 325 °C for the decomposition reaction \(\ce{C4H8⟶2C2H4}\) is 6.1 × 10−8 s−1, and the activation energy is 261 kJ per mole of C4H8. Determine the frequency factor for the reaction.
- Solution
-
S12.5.9
Using the Arrhenius equation allows me to find the frequency factor, A.
k=Ae-Ea/RT
k, Ea, R, and T are all known values. k, Ea, and T are given in the problem as 6.1x10-8, 261 kJ, and 598 K, respectively.
So, plugging them into the equation gives:
6.1x10-8 s-1=Ae(-261000 J)/(8.3145 J/mol)(598 K)
Take e(-261000 J)/(8.3145 J/mol)(598) and get 1.59 x 10-23. Divide k, 6.1 x 10-8, by 1.59 x 10-23 and get A=3.9 x 1015s-1
-
A12.5.9
\(\mathrm{3.9×10^{15}\:s^{−1}}\)
Q12.5.10
The rate constant for the decomposition of acetaldehyde (CH3CHO), to methane (CH4), and carbon monoxide (CO), in the gas phase is 1.1 × 10−2 L/mol/s at 703 K and 4.95 L/mol/s at 865 K. Determine the activation energy for this decomposition.
S12.5.10
The equation for relating the rate constant and activation energy of a reaction is the Arrhenius equation:
\[k = Ae^ {-\frac{E_a}{RT}}\]
When given two rate constants at two different temperatures but for the same reaction, the Arrhenius equation can be rewritten as:
\[ln (\frac{k_2}{k_1}) = \frac{E_a}{R} (\frac{1}{T_1} - \frac{1}{T_2})\]
In this problem, all the variables are given except for the Ea (activation energy).
k1 = 1.1 × 10−2 L/mol/s
T1 = 703 K
k2 = 4.95 L/mol/s
T2 = 865 K
R = 8.314 J/(mol K) (Ideal Gas Constant)
Now plug in all these values into the equation, and solve for Ea.
\[ln (\frac{4.95\frac{L}{mol×s}}{1.1 × 10^{-2}\frac{L}{mol×s}}) = \frac{E_a}{8.314 × 10^{-3}\frac{kJ}{mol×K}} (\frac{1}{703} - \frac{1}{865})\]
Ea = 190 kJ (2 sig figs)
Q12.5.11
An elevated level of the enzyme alkaline phosphatase (ALP) in the serum is an indication of possible liver or bone disorder. The level of serum ALP is so low that it is very difficult to measure directly. However, ALP catalyzes a number of reactions, and its relative concentration can be determined by measuring the rate of one of these reactions under controlled conditions. One such reaction is the conversion of p-nitrophenyl phosphate (PNPP) to p-nitrophenoxide ion (PNP) and phosphate ion. Control of temperature during the test is very important; the rate of the reaction increases 1.47 times if the temperature changes from 30 °C to 37 °C. What is the activation energy for the ALP–catalyzed conversion of PNPP to PNP and phosphate?
- Solution
-
43.0 kJ/mol
Q12.5.12
In terms of collision theory, to which of the following is the rate of a chemical reaction proportional?
- the change in free energy per second
- the change in temperature per second
- the number of collisions per second
- the number of product molecules
Q12.5.13
Hydrogen iodide, HI, decomposes in the gas phase to produce hydrogen, H2, and iodine, I2. The value of the rate constant, k, for the reaction was measured at several different temperatures and the data are shown here:
| Temperature (K) | k (M−1 s−1) |
|---|---|
| 555 | 6.23 × 10−7 |
| 575 | 2.42 × 10−6 |
| 645 | 1.44 × 10−4 |
| 700 | 2.01 × 10−3 |
What is the value of the activation energy (in kJ/mol) for this reaction?
- Solution
-
177 kJ/mol
Q12.5.14
The element Co exists in two oxidation states, Co(II) and Co(III), and the ions form many complexes. The rate at which one of the complexes of Co(III) was reduced by Fe(II) in water was measured. Determine the activation energy of the reaction from the following data:
| T (K) | k (s−1) |
|---|---|
| 293 | 0.054 |
| 298 | 0.100 |
Q12.5.15
The hydrolysis of the sugar sucrose to the sugars glucose and fructose,
\[\ce{C12H22O11 + H2O ⟶ C6H12O6 + C6H12O6}\nonumber \]
follows a first-order rate equation for the disappearance of sucrose: Rate = k[C12H22O11] (The products of the reaction, glucose and fructose, have the same molecular formulas but differ in the arrangement of the atoms in their molecules.)
- In neutral solution, k = 2.1 × 10−11 s−1 at 27 °C and 8.5 × 10−11 s−1 at 37 °C. Determine the activation energy, the frequency factor, and the rate constant for this equation at 47 °C (assuming the kinetics remain consistent with the Arrhenius equation at this temperature).
- When a solution of sucrose with an initial concentration of 0.150 M reaches equilibrium, the concentration of sucrose is 1.65 × 10−7 M. How long will it take the solution to reach equilibrium at 27 °C in the absence of a catalyst? Because the concentration of sucrose at equilibrium is so low, assume that the reaction is irreversible.
- Why does assuming that the reaction is irreversible simplify the calculation in part (b)?
- Solution
-
Ea = 108 kJ
A = 2.0 × 108 s−1
k = 3.2 × 10−10 s−1
(b) 1.81 × 108 h or 7.6 × 106 day. (c) Assuming that the reaction is irreversible simplifies the calculation because we do not have to account for any reactant that, having been converted to product, returns to the original state.
Q12.5.16
Use the PhET Reactions & Rates interactive simulation to simulate a system. On the “Single collision” tab of the simulation applet, enable the “Energy view” by clicking the “+” icon. Select the first \(A+BC⟶AB+C\) reaction (A is yellow, B is purple, and C is navy blue). Using the “straight shot” default option, try launching the A atom with varying amounts of energy. What changes when the Total Energy line at launch is below the transition state of the Potential Energy line? Why? What happens when it is above the transition state? Why?
Q12.5.17
Use the PhET Reactions & Rates interactive simulation to simulate a system. On the “Single collision” tab of the simulation applet, enable the “Energy view” by clicking the “+” icon. Select the first \(A+BC⟶AB+C\) reaction (A is yellow, B is purple, and C is navy blue). Using the “angled shot” option, try launching the A atom with varying angles, but with more Total energy than the transition state. What happens when the A atom hits the BC molecule from different directions? Why?
- Solution
-
The A atom has enough energy to react with BC; however, the different angles at which it bounces off of BC without reacting indicate that the orientation of the molecule is an important part of the reaction kinetics and determines whether a reaction will occur.
12.6: Reaction Mechanisms
Q12.6.1
Why are elementary reactions involving three or more reactants very uncommon?
Q12.6.2
In general, can we predict the effect of doubling the concentration of A on the rate of the overall reaction \(A+B⟶C\) ? Can we predict the effect if the reaction is known to be an elementary reaction?
- Solution
-
Add texts here. Do not delete this text first.
No. In general, for the overall reaction, we cannot predict the effect of changing the concentration without knowing the rate equation. Yes. If the reaction is an elementary reaction, then doubling the concentration of A doubles the rate.
Q12.6.3
Phosgene, COCl2, one of the poison gases used during World War I, is formed from chlorine and carbon monoxide. The mechanism is thought to proceed by:
| step 1: | Cl + CO → COCl |
| step 2: | COCl + Cl2→ COCl2 + Cl |
- Write the overall reaction equation.
- Identify any reaction intermediates.
- Identify any intermediates.
Q12.6.4
Define these terms:
- unimolecular reaction
- bimolecular reaction
- elementary reaction
- overall reaction
Q12.6.5
What is the rate equation for the elementary termolecular reaction \(A+2B⟶\ce{products}\)? For \(3A⟶\ce{products}\)?
- Solution
-
Add texts here. Do not delete this text first.
We are given that both of these reactions are elementary termolecular. The molecularity of a reaction refers to the number of reactant particles that react together with the proper and energy and orientation. Termolecular reactions have three atoms to collide simultaneously. As it is termolecular, and there are no additional reactants aside from the three given in each reaction, there are no intermediate reactions. The rate law for elementary reactions is determined by the stoichiometry of the reaction without needed experimental data.
The basic rate form for the elementary step is what follows:
\(rate= {k} \cdot {reactant \ 1}^{i} \cdot {reactant \ 2}^{ii} \cdot ... \) Where i and ii are the stochiometric coefficient from reactant 1 and 2 respectively.
For: \(3A \rightarrow products \)
\({k} \cdot {A}^3 = rate\)
For: \(A + 2B \rightarrow products \)
\({k} \cdot {[A]} \cdot {[B]}^2 = rate\)
Note that the order of these reactions are both three.
- Answer
-
Add texts here. Do not delete this text first.
Rate = k[A][B]2; Rate = k[A]3
Q12.6.6
Given the following reactions and the corresponding rate laws, in which of the reactions might the elementary reaction and the overall reaction be the same?
(a) \(\ce{Cl2 + CO ⟶ Cl2CO}\)
\(\ce{rate}=k\ce{[Cl2]^{3/2}[CO]}\)
(b) \(\ce{PCl3 + Cl2 ⟶ PCl5}\)
\(\ce{rate}=k\ce{[PCl3][Cl2]}\)
(c) \(\ce{2NO + H2 ⟶ N2 + H2O}\)
\(\ce{rate}=k\ce{[NO][H2]}\)
(d) \(\ce{2NO + O2 ⟶ 2NO2}\)
\(\ce{rate}=k\ce{[NO]^2[O2]}\)
(e) \(\ce{NO + O3 ⟶ NO2 + O2}\)
\(\ce{rate}=k\ce{[NO][O3]}\)
- Solution
-
Add texts here. Do not delete this text first.
An elementary reaction is a chemical reaction in which the reactants directly form products in a single step. In another words, the rate law for the overall reaction is same as experimentally found rate law. Out of 5 options, option (b),(d), and (e) are such reactions
Q12.6.7
Write the rate equation for each of the following elementary reactions:
- \(\ce{O3 \xrightarrow{sunlight} O2 + O}\)
- \(\ce{O3 + Cl ⟶ O2 + ClO}\)
- \(\ce{ClO + O⟶ Cl + O2}\)
- \(\ce{O3 + NO ⟶ NO2 + O2}\)
- \(\ce{NO2 + O ⟶ NO + O2}\)
- Solution
-
Add texts here. Do not delete this text first.
Rate equations are dependent on the reactants and not the products.
The rate law of a reaction can be found using a rate constant (which is found experimentally), and the initial concentrations of reactants.
A general solution for the equation
\(aA + bB \rightarrow cC + dD\)
is \(rate = k[A]^{m}[B]^{n}\) where m and n are reaction orders.
However, reaction orders are found experimentally, and since we do not have experimental data for these reactions, we can disregard that part of the equation.
To find the rate laws, all we have to do is plug the reactants into the rate formula. This is only due to the case that these are elementary reactions. Further reading on elementary reactions can be found on Libre Texts.
a. O3 ⟶ O2 + O
To write this reaction's rate equation, only focus on the reactant(s) and its/their concentration and multiplying that by a rate constant, "k".
Rate = k[O3]
b. O3 + Cl ⟶ O2 + ClO
To write this reaction's rate equation, only focus on the reactant(s) and its/their concentration and multiplying that by a rate constant, "k".
Rate = k[O3][Cl]
c. ClO + O ⟶ Cl + O2
To write this reaction's rate equation, only focus on the reactant(s) and its/their concentration and multiplying that by a rate constant, "k".
Rate = k[ClO][O]
d. O3 + NO ⟶ NO2 + O2
To write this reaction's rate equation, only focus on the reactant(s) and its/their concentration and multiplying that by a rate constant, "k".
Rate = k[O3][NO]
e. NO2 + O ⟶ NO + O2
To write this reaction's rate equation, only focus on the reactant(s) and its/their concentration and multiplying that by a rate constant, "k".
Rate = k[NO2][O]
- Answer
-
Add texts here. Do not delete this text first.
(a) Rate1 = k[O3]; (b) Rate2 = k[O3][Cl]; (c) Rate3 = k[ClO][O]; (d) Rate2 = k[O3][NO]; (e) Rate3 = k[NO2][O]
Q12.6.8
Nitrogen(II) oxide, NO, reacts with hydrogen, H2, according to the following equation:
\[\ce{2NO + 2H2 ⟶ N2 + 2H2O}\nonumber \]
What would the rate law be if the mechanism for this reaction were:
\[\ce{2NO + H2 ⟶ N2 + H2O2\:(slow)}\nonumber \]
\[\ce{H2O2 + H2 ⟶ 2H2O\:(fast)}\nonumber \]
The rate law of the mechanism is determined by the slow step of the reaction. Since the slow step is an elementary step, the rate law can be drawn from the coefficients of the chemical equation. So therefore, the rate law is as follows: rate=k[NO]2[H2]. Since both NO and H2 are reactants in the overall reaction (therefore are not intermediates in the reaction), no further steps have to be done to determine the rate law.
Q12.6.9
Consider the reaction
CH4 + Cl2 → CH3Cl + HCl (occurs under light)
The mechanism is a chain reaction involving Cl atoms and CH3 radicals. Which of the following steps does not terminate this chain reaction?
- CH3 + Cl → CH3CI
- CH3 + HCl → CH4 + Cl
- CH3 + CH3 → C2H2
- Cl + Cl → Cl2
- Solution
-
Add texts here. Do not delete this text first.
Chain reactions involve reactions that create products necessary for more reactions to occur. In this case, a reaction step will continue the chain reaction if a radical is generated. Radicals are highly reactive particles, so more reactions in the chain will take place as long as they are present. The chlorine is considered a free radical as it has an unpaired electron; for this reason it is very reactive and propagates a chain reaction. It does so by taking an electron from a stable molecule and making that molecule reactive, and that molecule goes on to react with stable species, and in that manner a long series of "chain" reactions are initiated. A chlorine radical will continue the chain by completing the following reaction:
\({Cl \cdot}+{CH_4} \rightarrow {CH_3 \cdot}+{HCl} \)
The \({CH_3}\) generated by this reaction can then react with other species, continuing to propagate the chain reaction.
Option 1 is incorrect because the only species it produces is \({CH_3Cl}\), a product in the overall reaction that is unreactive. This terminates the chain reaction because it fails to produce any \(Cl\) or \(CH_3\) radicals that are necessary for further propagating the overall reaction.
Option 2 is the correct answer because it produces a \(Cl\) radical. This \(Cl\) radical can continue the chain by colliding with \(CH_4\) molecules.
Option 3 is incorrect because it fails to produce a radical capable of continuing the chain.
Option 4 is incorrect because it produces \(Cl_2\), a molecule that does not react unless additional light is supplied. Therefore, this step breaks the chain.
- Answer
-
Add texts here. Do not delete this text first.
Answer: Option 2: \({CH_3}+{HCl} \rightarrow {CH_4}+{Cl}\)
Q12.6.10
Experiments were conducted to study the rate of the reaction represented by this equation.
\[\ce{2NO}(g)+\ce{2H2}(g)⟶\ce{N2}(g)+\ce{2H2O}(g)\nonumber \]
Initial concentrations and rates of reaction are given here.
| Experiment | Initial Concentration [NO] (mol/L) | Initial Concentration, [H2] (mol/L) | Initial Rate of Formation of N2 (mol/L min) |
|---|---|---|---|
| 1 | 0.0060 | 0.0010 | 1.8 × 10−4 |
| 2 | 0.0060 | 0.0020 | 3.6 × 10−4 |
| 3 | 0.0010 | 0.0060 | 0.30 × 10−4 |
| 4 | 0.0020 | 0.0060 | 1.2 × 10−4 |
Consider the following questions:
- Determine the order for each of the reactants, NO and H2, from the data given and show your reasoning.
- Write the overall rate law for the reaction.
- Calculate the value of the rate constant, k, for the reaction. Include units.
- For experiment 2, calculate the concentration of NO remaining when exactly one-half of the original amount of H2 had been consumed.
- The following sequence of elementary steps is a proposed mechanism for the reaction.
Step 1: \(\ce{NO + NO ⇌ N2O2}\)
Step 2: \(\ce{N2O2 + H2 ⇌ H2O + N2O}\)
Step 3: \(\ce{N2O + H2 ⇌ N2 + H2O}\)
Based on the data presented, which of these is the rate determining step? Show that the mechanism is consistent with the observed rate law for the reaction and the overall stoichiometry of the reaction.
S12.6.10
1. i) Find the order for [NO] by using experiment 3 and 4 where [H2] is constant
Notice that [NO] doubles from experiment 3 to 4 and the rate quadruples. So the order for [NO] is 2
ii) Find the order for [H2] by using experiment 1 and 2 where [NO] is constant
Notice that [H2] doubles from experiment 1 to 2 and the rate doubles as well. So the order for [H2] is 1
2. Put in the order for each product as the exponents for the corresponding reactant.
\(rate = k [NO]^2 [H_2]\)
3. Put in the concentrations and the rate from one of the experiments into the rate law and solve for k. (Here, experiement 1 is used but any of them will work)
\(rate = k [NO]^2 [H_2]\) \(.00018 = k [.006]^2 [.001]\) \(k = 5000 M^{-2}s^{-1}\)
4. Plug in values for experiment 2 into the rate law equation and solve for the concentration of NO
\(.00036=5000[NO]^2[.001]\) \([NO]^2= 7.2 x 10^{-5}\) \([NO] = .0085 M\)
5. Write the rate laws for each step and then see which matches the rate law we found in question 2. The rate determining step (the slow step) is the one that gives the rate for the overall reaction. Because of this, only those concentrations will influence the overall reaction, contrary to what we would believe if we just looked at the overall reaction.
Step 1: \(NO + NO \rightleftharpoons N_2O_2\)
\(rate =k_1[NO]^2\) This rate law is not the same as the one we calculate in question 2 so this can not be the rate determining step
Step 2: \(N_2O_2+H_2 \rightleftharpoons N_2O + N_2O\)
\(rate = k_2[N_2O_2][H_2]\)
Since \(N_2O_2\) is an intermediate you must replace it in the rate law equation. Intermediates can not be in the rate law because they do not appear in the overall reaction. Here you can take the reverse of equation 1 (k-1) and substitute the other side (the reactants of equation 1) for the intermediate in the rate law equation.
\[rate_1 = rate_{-1}\nonumber \]
\[k_1[NO]^2 = k_{-1}[N_2O_2]\nonumber \]
\[[N_2O_2] = \frac{k_1[NO]^2}{k_{-1}}\nonumber \]
\(rate= \frac{k_2k_{1}[NO]^2[H_2]}{k_{-1}}\)
Overall: \(rate={k[NO]^2[H_2]}\) This is the same so it is the rate determining step.
So \(N_2O_2+H_2 \rightleftharpoons N_2O + N_2O\) is the rate determining step step.
- Answer
-
Add texts here. Do not delete this text first.
(a) NO: 2
\(\ce {H2}\) : 1
(b) Rate = k [NO]2[H2];
(c) k = 5.0 × 103 mol−2 L−2 min−1;
(d) 0.0050 mol/L;
(e) Step II is the rate-determining step.
Q12.6.11
The reaction of CO with Cl2 gives phosgene (COCl2), a nerve gas that was used in World War I. Use the mechanism shown here to complete the following exercises:
- \(\ce{Cl2}(g)⇌\ce{2Cl}(g)\) (fast, k1 represents the forward rate constant, k−1 the reverse rate constant)
- \(\ce{CO}(g)+\ce{Cl}(g)⟶\ce{COCl}(g)\) (slow, k2 the rate constant)
- \(\ce{COCl}(g)+\ce{Cl}(g)⟶\ce{COCl2}(g)\) (fast, k3 the rate constant)
- Write the overall reaction.
- Identify all intermediates.
- Write the rate law for each elementary reaction.
- Write the overall rate law expression.
- Solution
-
Add texts here. Do not delete this text first.
1. To write the overall reaction you have to identify the intermediates and leave them out. The easiest way to do this is to write out all the products and reactants and cross out anything that is on both sides.
Cl2(g) + CO(g) + 2Cl(g) +COCl(g) ⇒ 2Cl(g) + COCl(g) + COCl2(g)
In this you will cross out the 2Cl(g) molecules and the COCl(g). What is left after that is the overall reaction.
Cl2(g) + CO(g) ⇒ + COCl2(g)
2. For part two you will just list the intermediates that you crossed out.
Cl and COCl are intermediates
3. Each rate law will be the rate equal to the rate constant times the concentrations of the reactants
reaction 1
(forward) rate=k1[Cl2] ( reverse) rate=k-1[Cl]
reaction 2
rate=k2[CO][Cl]
Reaction 3
rate=k3[COCl][Cl]
4. The overall rate law is based off the slowest step (step #2), since it is the rate determining step, but Cl is present in that rate law so we have to replace it with an equivalent that does not contain an intermediate. To do this you use the equilibrium since the rates are the same you can set up the rate laws of the forward and reverse equal to each other.
k1[Cl2] = k-1[Cl]
[Cl]= k1[Cl2]/k-1
rate=k2[CO]k1[Cl2]/k-1
rate=k°[CO][Cl2]
Steps to replacing and intermediate
- Set the forward and reverse reaction equal to each other using separate constants
- Solve for the intermediate using algebra
- Plug into the rate determining formula
- All the k's will be condensed into a K prime constant
12.7: Catalysis
Q12.7.1
Account for the increase in reaction rate brought about by a catalyst.
Q12.7.2
Compare the functions of homogeneous and heterogeneous catalysts.
Q12.7.3
Consider this scenario and answer the following questions: Chlorine atoms resulting from decomposition of chlorofluoromethanes, such as CCl2F2, catalyze the decomposition of ozone in the atmosphere. One simplified mechanism for the decomposition is: \[\ce{O3 \xrightarrow{sunlight} O2 + O}\\ \ce{O3 + Cl ⟶ O2 + ClO}\\ \ce{ClO + O ⟶ Cl + O2}\nonumber \]
- Explain why chlorine atoms are catalysts in the gas-phase transformation: \[\ce{2O3⟶3O2}\nonumber \]
- Nitric oxide is also involved in the decomposition of ozone by the mechanism: \[\ce{O3 \xrightarrow{sunlight} O2 + O\\ O3 + NO ⟶ NO2 + O2\\ NO2 + O ⟶ NO + O2}\nonumber \]
Is NO a catalyst for the decomposition? Explain your answer.
Q12.7.4
For each of the following pairs of reaction diagrams, identify which of the pair is catalyzed:
(a)
Q12.7.5
For each of the following pairs of reaction diagrams, identify which of the pairs is catalyzed:
(a)
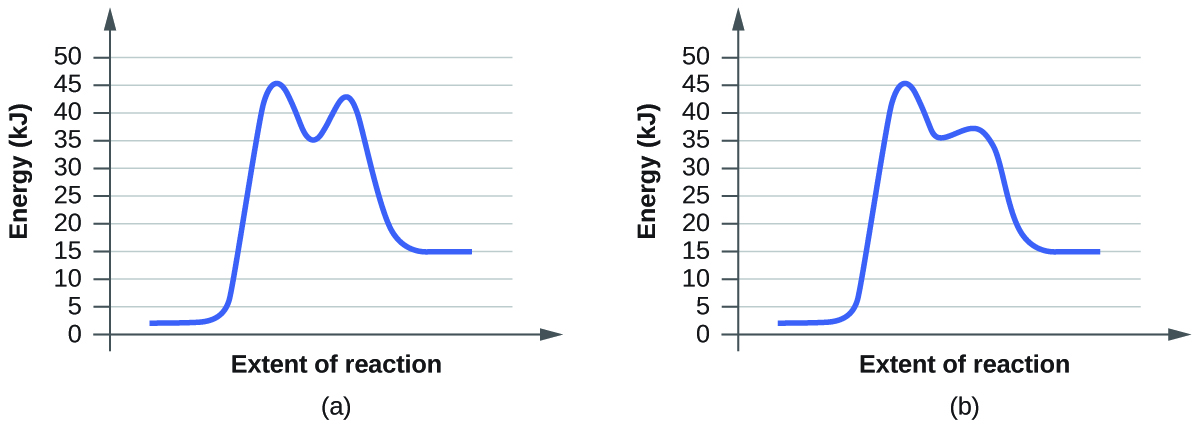
(b)
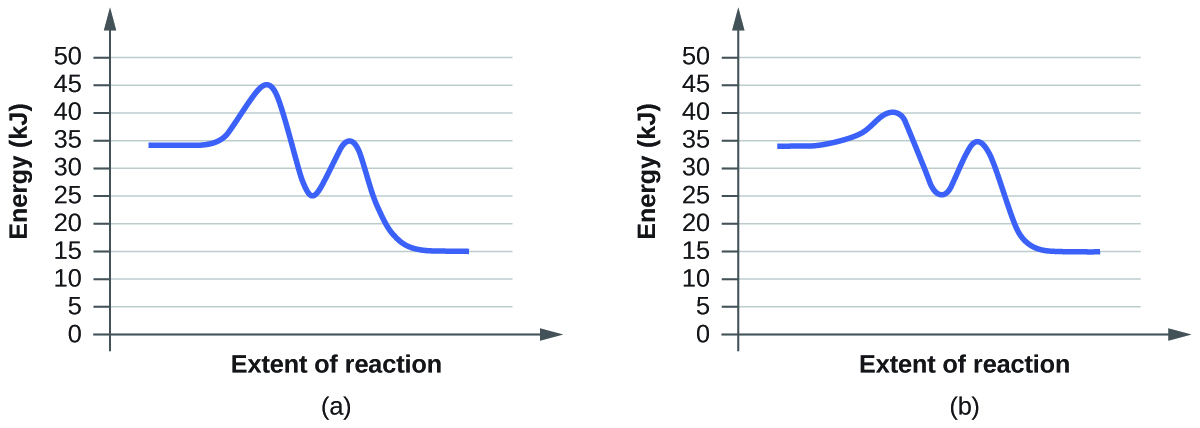
Q12.7.6
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
(a)
(b)
Q12.7.7
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
(a)

(b)
Q12.7.8
- Based on the diagrams in Question Q12.7.6, which of the reactions has the fastest rate? Which has the slowest rate?
- Based on the diagrams in Question Q12.7.7, which of the reactions has the fastest rate? Which has the slowest rate?



![{\ displaystyle t_ {\ frac {1} {2}} = {\ frac {1} {k {\ ce {[A] 0}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b25f9b5e7675b3e3f9027241a74ec2d751810c43)