4.E: Stoichiometry ya athari za kemikali (Mazoezi)
- Page ID
- 176932
4.1: Kuandika na kusawazisha Ulinganisho wa Kemikali
Q4.1.1
Ina maana gani kusema equation ni uwiano? Kwa nini ni muhimu kwa equation kuwa na usawa?
S4.1.1
equation ni uwiano wakati idadi sawa ya kila kipengele ni kuwakilishwa kwenye pande reactant na bidhaa. Ulinganifu lazima uwe na usawa wa kutafakari kwa usahihi sheria ya uhifadhi wa suala.
Q4.1.2
Fikiria molekuli, kamili ionic, na equations wavu ionic.
- Ni tofauti gani kati ya aina hizi za equations?
- Katika hali gani ingekuwa equations kamili na wavu ionic kwa mmenyuko kuwa sawa?
Q4.1.3
Mizani equations zifuatazo:
- \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{HCl}(aq)\)
- \(\ce{Cu}(s)+\ce{HNO3}(aq)\rightarrow \ce{Cu(NO3)2}(aq)+\ce{H2O}(l)+\ce{NO}(g)\)
- \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{HI}(s)\)
- \(\ce{Fe}(s)+\ce{O2}(g)\rightarrow \ce{Fe2O3}(s)\)
- \(\ce{Na}(s)+\ce{H2O}(l)\rightarrow \ce{NaOH}(aq)+\ce{H2}(g)\)
- \(\ce{(NH4)2Cr2O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{H2O}(g)\)
- \(\ce{P4}(s)+\ce{Cl2}(g)\rightarrow \ce{PCl3}(l)\)
- \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{Cl2}(g)\)
S4.1.3
- \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{2HCl}(aq)\);
- \(\ce{3Cu}(s)+\ce{8HNO3}(aq)\rightarrow \ce{3Cu(NO3)2}(aq)+\ce{4H2O}(l)+\ce{2NO}(g)\);
- \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{2HI}(s)\);
- \(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\);
- \(\ce{2Na}(s)+\ce{2H2O}(l)\rightarrow \ce{2NaOH}(aq)+\ce{H2}(g)\);
- \(\ce{(NH4)2Cr52O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{4H2O}(g)\);
- \(\ce{P4}(s)+\ce{6Cl2}(g)\rightarrow \ce{4PCl3}(l)\);
- \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{2Cl2}(g)\)
Q4.1.4
Mizani equations zifuatazo:
- \(\ce{Ag}(s)+\ce{H2S}(g)+\ce{O2}(g)\rightarrow \ce{Ag2S}(s)+\ce{H2O}(l)\)
- \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \ce{P4O10}(s)\)
- \(\ce{Pb}(s)+\ce{H2O}(l)+\ce{O2}(g)\rightarrow \ce{Pb(OH)2}(s)\)
- \(\ce{Fe}(s)+\ce{H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{H2}(g)\)
- \(\ce{Sc2O3}(s)+\ce{SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\)
- \(\ce{Ca3(PO4)2}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{Ca(H2PO4)2}(aq)\)
- \(\ce{Al}(s)+\ce{H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{H2}(g)\)
- \(\ce{TiCl4}(s)+\ce{H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{HCl}(g)\)
S4.1.4
- \(\ce{4Ag}(s)+\ce{2H2S}(g)+\ce{O2}(g)\rightarrow \ce{2Ag2S}(s)+\ce{2H2O}(l)\)
- \(\ce{P4}(s)+\ce{5O2}(g)\rightarrow \ce{P4O10}(s)\)
- \(\ce{2Pb}(s)+\ce{2H2O}(l)+\ce{O2}(g)\rightarrow \ce{2Pb(OH)2}(s)\)
- \(\ce{3Fe}(s)+\ce{4H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{4H2}(g)\)
- \(\ce{Sc2O3}(s)+\ce{3SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\)
- \(\ce{Ca3(PO4)2}(aq)+\ce{4H3PO4}(aq)\rightarrow \ce{3Ca(H2PO4)2}(aq)\)
- \(\ce{2Al}(s)+\ce{3H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{3H2}(g)\)
- \(\ce{TiCl4}(s)+\ce{2H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{4HCl}(g)\)
Q4.1.5
Andika uwiano Masi equation kuelezea kila moja ya yafuatayo athari kemikali.
- Mango calcium carbonate ni joto na hutengana na oksidi kalsiamu imara na gesi kaboni dioksidi.
- Butane ya gesi, C 4 H 10, humenyuka na gesi ya oksijeni ya diatomiki ili kuzalisha dioksidi kaboni ya gesi na mvuke wa maji.
- Ufumbuzi wa maji ya kloridi magnesiamu na hidroksidi ya sodiamu huguswa ili kuzalisha hidroksidi
- Mvuke wa maji humenyuka na chuma cha sodiamu ili kuzalisha hidroksidi imara ya s
S4.1.5
- \(\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(g)\);
- \(\ce{2C4H10}(g)+\ce{13O2}(g)\rightarrow \ce{8CO2}(g)+\ce{10H2O}(g)\);
- \(\ce{MgCl2}(aq)+\ce{2NaOH}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{2NaCl}(aq)\);
- \(\ce{2H2O}(g)+\ce{2Na}(s)\rightarrow \ce{2NaOH}(s)+\ce{H2}(g)\)
Q4.1.6
Andika equation uwiano kuelezea kila moja ya athari zifuatazo kemikali.
- Kloreti ya potasiamu imara, kClO 3, hutengana ili kuunda kloridi ya potasiamu imara na gesi ya oksijeni ya diatomiki.
- Mango chuma alumini humenyuka na iodini imara diatomic kuunda imara Al 2 I 6.
- Wakati kloridi imara ya sodiamu imeongezwa kwa asidi ya sulfuriki yenye maji, gesi ya hidrojeni ya hidrojeni na sulfate yenye maji yanazalishwa
- Ufumbuzi wa maji ya asidi fosforasi na hidroksidi ya potasiamu huguswa ili kuzalisha phosphate ya potasiamu yenye maji
Q4.1.7
Mara nyingi fireworks rangi huhusisha utengano wa nitrati ya bariamu na kloreti ya potasiamu na mmenyuko wa madini magnesiamu, alumini, na chuma na oksijeni.
- Andika kanuni za nitrati ya bariamu na klorate ya potasiamu.
- Uharibifu wa kloreti ya potasiamu imara husababisha kuundwa kwa kloridi imara ya potasiamu na gesi ya oksijeni ya diatomiki. Andika equation kwa majibu.
- Uharibifu wa nitrati imara ya bariamu husababisha kuundwa kwa oksidi ya bariamu imara, gesi ya nitrojeni ya diatomiki, na gesi ya oksijeni ya diatomiki. Andika equation kwa majibu.
- Andika equations tofauti kwa athari za metali imara magnesiamu, alumini, na chuma na gesi diatomic oksijeni ili kuzalisha oksidi za chuma zinazofanana. (Fikiria oksidi ya chuma ina Fe 3+ ions.)
Q4.1.7
- Ba (NO 3) 2, kClo 3;
- \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\);
- \(\ce{2Ba(NO3)2}(s)\rightarrow \ce{2BaO}(s)+\ce{2N2}(g)+\ce{5O2}(g)\);
- \(\ce{2Mg}(s)+\ce{O2}(g)\rightarrow \ce{2MgO}(s)\);\(\ce{4Al}(s)+\ce{3O2}(g)\rightarrow \ce{2Al2O3}(g)\);\(\ce{4Fe}(s)+\ce{3O2}(g)\rightarrow \ce{2Fe2O3}(s)\)
Q4.1.8
Jaza tupu na formula moja ya kemikali kwa kiwanja cha covalent ambacho kitasanisha usawa:
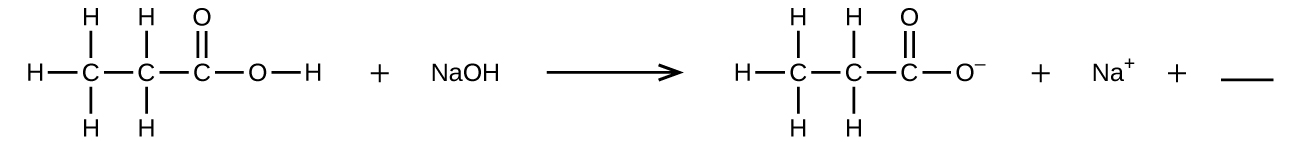
Q4.1.9
Fluoride ya hidrojeni yenye maji machafu (asidi hydrofluoric) hutumiwa kutengeneza kioo na kuchambua madini kwa maudhui yao ya silicon. Fluoride ya hidrojeni pia itaitikia na mchanga (silicon dioxide).
- Andika equation kwa mmenyuko wa dioksidi imara ya silicon na asidi hidrofluoriki ili kuzalisha tetrafluoride ya gesi ya silicon na maji ya kioevu.
- Fluorite ya madini (fluoride ya kalsiamu) hutokea sana huko Illinois. Mango calcium fluoride pia inaweza kuwa tayari na majibu ya ufumbuzi wa maji ya kloridi kalsiamu na fluoride sodiamu, kutoa maji ya sodium chloride kama bidhaa nyingine. Andika equations kamili na wavu ionic kwa mmenyuko huu.
S4.1.9
- \(\ce{4HF}(aq)+\ce{SiO2}(s)\rightarrow \ce{SiF4}(g)+\ce{2H2O}(l)\);
- kamili ionic equation:\(\ce{2Na+}(aq)+\ce{2F-}(aq)+\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)\rightarrow \ce{CaF2}(s)+\ce{2Na+}(aq)+\ce{2Cl-}(aq)\), wavu ionic equation:\(\ce{2F-}(aq)+\ce{Ca^2+}(aq)\rightarrow \ce{CaF2}(s)\)
Q4.1.10
Mchakato wa riwaya wa kupata magnesiamu kutoka maji ya bahari unahusisha athari kadhaa. Andika usawa wa kemikali kwa kila hatua ya mchakato.
- Hatua ya kwanza ni kuharibika kwa carbonate ya kalsiamu imara kutoka seashells ili kuunda oksidi ya kalsiamu imara na dioksidi kaboni ya gesi.
- Hatua ya pili ni malezi ya hidroksidi ya kalsiamu imara kama bidhaa pekee kutoka kwa mmenyuko wa oksidi ya kalsiamu imara na maji ya kioevu.
- Hidroksidi ya kalsiamu imara huongezwa kwa maji ya bahari, ikitikia na kloridi ya magnesiamu iliyoharibika ili kuzalisha hidroksidi ya magnesiamu imara na
- Hidroksidi ya magnesiamu imara imeongezwa kwenye suluhisho la asidi hidrokloriki, huzalisha kloridi ya magnesiamu iliyoharib
- Hatimaye, kloridi ya magnesiamu imeyeyuka na electrolyzed ili kutoa chuma cha magnesiamu kioevu na gesi ya klorini
Q4.1.11
Kutoka kwa usawa wa molekuli ya usawa, andika equations kamili ya ionic na wavu ionic kwa yafuatayo:
- \(\ce{K2C2O4}(aq)+\ce{Ba(OH)2}(aq)\rightarrow \ce{2KOH}(aq)+\ce{BaC2O2}(s)\)
- \(\ce{Pb(NO3)2}(aq)+\ce{H2SO4}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2HNO3}(aq)\)
- \(\ce{CaCO3}(s)+\ce{H2SO4}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\)
S4.1.11
- \[\ce{2K+}(aq)+\ce{C2O4^2-}(aq)+\ce{Ba^2+}(aq)+\ce{2OH-}(aq)\rightarrow \ce{2K+}(aq)+\ce{2OH-}(aq)+\ce{BaC2O4}(s)\hspace{20px}\ce{(complete)}\]\[\ce{Ba^2+}(aq)+\ce{C2O4^2-}(aq)\rightarrow \ce{BaC2O4}(s)\hspace{20px}\ce{(net)}\]
- \[\ce{Pb^2+}(aq)+\ce{2NO3-}(aq)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2H+}(aq)+\ce{2NO3-}(aq)\hspace{20px}\ce{(complete)}\]\[\ce{Pb^2+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{PbSO4}(s)\hspace{20px}\ce{(net)}\]
- \[\ce{CaCO3}(s)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\hspace{20px}\ce{(complete)}\]\[\ce{CaCO3}(s)+\ce{2H+}(aq)+\ce{SO4^2-}(aq)\rightarrow \ce{CaSO4}(s)+\ce{CO2}(g)+\ce{H2O}(l)\hspace{20px}\ce{(net)}\]
4.2: Kuainisha athari za Kemikali
Q4.2.1
Tumia equations zifuatazo kujibu maswali tano ijayo:
- \(\ce{H2O}(s)\rightarrow \ce{H2O}(l)\)
- \(\ce{Na+}(aq)+\ce{Cl-}(aq)\ce{Ag+}(aq)+\ce{NO3-}(aq) \rightarrow \ce{AgCl}(s)+\ce{Na+}(aq)+\ce{NO3-}(aq)\)
- \(\ce{CH3OH}(g)+\ce{O2}(g)\rightarrow \ce{CO2}(g)+\ce{H2O}(g)\)
- \(\ce{2H2O}(l)\rightarrow \ce{2H2}(g)+\ce{O2}(g)\)
- \(\ce{H+}(aq)+\ce{OH-}(aq)\rightarrow \ce{H2O}(l)\)
- Ambayo equation inaelezea mabadiliko ya kimwili?
- Ambayo equation kubainisha reactants na bidhaa za mmenyuko mwako?
- Ambayo equation si uwiano?
- Ambayo ni wavu ionic equation?
S4.2.1
a.) i.\(H_2O (solid) → H_2O(liquid)\)
b.) iii.
c.) iii. \(\ce{2CH3OH}(g)+\ce{3O2}(g)\rightarrow \ce{2CO2}(g)+\ce{4H2O}(g)\)
d.) v.
Q4.2.2
Eleza aina gani, au aina, za majibu kila moja ya yafuatayo inawakilisha:
- \(\ce{Ca}(s)+\ce{Br2}(l)\rightarrow \ce{CaBr2}(s)\)
- \(\ce{Ca(OH)2}(aq)+\ce{2HBr}(aq)\rightarrow \ce{CaBr2}(aq)+\ce{2H2O}(l)\)
- \(\ce{C6H12}(l)+\ce{9O2}(g)\rightarrow \ce{6CO2}(g)+\ce{6H2O}(g)\)
S4.2.2
kupunguza oxidation (kuongeza); asidi-msingi (neutralization); kupunguza oxidation (mwako)
<
Q4.2.3
Eleza aina gani, au aina, za majibu kila moja ya yafuatayo inawakilisha:
- \(\ce{H2O}(g)+\ce{C}(s)\rightarrow \ce{CO}(g)+\ce{H2}(g)\)
- \(\ce{2KClO3}(s)\rightarrow \ce{2KCl}(s)+\ce{3O2}(g)\)
- \(\ce{Al(OH)3}(aq)+\ce{3HCl}(aq)\rightarrow \ce{AlBr3}(aq)+\ce{3H2O}(l)\)
- \(\ce{Pb(NO3)2}(aq)+\ce{H2SO4}(aq)\rightarrow \ce{PbSO4}(s)+\ce{2HNO3}(aq)\)
Q4.2.4
Fedha inaweza kutengwa na dhahabu kwa sababu fedha hupasuka katika asidi ya nitriki ilhali dhahabu haifai. Je, uharibifu wa fedha katika asidi ya nitriki ni mmenyuko wa asidi-msingi au mmenyuko wa kupunguza oxidation? Eleza jibu lako.
S4.2.4
Ni mmenyuko wa kupunguza oxidation kwa sababu hali ya oxidation ya mabadiliko ya fedha wakati wa majibu.
Q4.2.5
Kuamua majimbo ya oxidation ya vipengele katika misombo ifuatayo:
- NaI
- GdCl 3
- Lino 3
- H 2 Se
- mg 2 Si
- Bro 2, superoxide ya rubidium
- HF
Q4.2.6
Kuamua hali ya oxidation ya vipengele katika misombo iliyoorodheshwa. Hakuna misombo yenye oksijeni ni peroxides au superoxides.
- H 3 PO 4
- Al (OH) 3
- SEO 2
- KNO 2
- Katika 2 S 3
- P 4 HADI 6
S4.2.6
H +1, P +5, O -1; Al +3, H +1, O -2; Se +4, O -2; K +1, N +3, O -2; Katika +3, S -1; P +3, O -2
Q4.2.7
Kuamua hali ya oxidation ya vipengele katika misombo iliyoorodheshwa. Hakuna misombo yenye oksijeni ni peroxides au superoxides.
- H 2 HIVYO 4
- Car (OH) 2
- BroH
- Clno 2
- TiCl 4
- NaH
S4.2.7
- H 1+, O 2 -, S 6+
- H 1+, O 2 -, Car +2
- H 1+, O 2 -, Br 1+
- O 2 -, Cl 1-, N 5+
- Cl 1-, Ti 4+
- H 1+, Na 1-
Q4.2.8
Weka zifuatazo kama athari za asidi-msingi au athari za kupunguza oxidation:
- \(\ce{Na2S}(aq)+\ce{2HCl}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{H2S}(g)\)
- \(\ce{2Na}(s)+\ce{2HCl}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{H2}(g)\)
- \(\ce{Mg}(s)+\ce{Cl2}(g)\rightarrow \ce{MgCl2}(s)\)
- \(\ce{MgO}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{H2O}(l)\)
- \(\ce{K3P}(s)+\ce{2O2}(g)\rightarrow \ce{K3PO4}(s)\)
- \(\ce{3KOH}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{K3PO4}(aq)+\ce{3H2O}(l)\)
S4.2.9
asidi-msingi; kupunguza oxidation: Na ni oxidized, H + imepunguzwa; kupunguza oxidation: Mg ni oxidized, Cl 2 imepunguzwa; asidi-msingi; kupunguza oxidation: P 3 - ni oxidized, O 2 imepunguzwa; asidi-msingi
Q4.2.10
Kutambua atomi ambazo ni oxidized na kupunguzwa, mabadiliko katika hali oxidation kwa kila, na oxidizing na kupunguza mawakala katika kila moja ya equations zifuatazo:
- \(\ce{Mg}(s)+\ce{NiCl2}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{Ni}(s)\)
- \(\ce{PCl3}(l)+\ce{Cl2}(g)\rightarrow \ce{PCl5}(s)\)
- \(\ce{C2H4}(g)+\ce{3O2}(g)\rightarrow \ce{2CO2}(g)+\ce{2H2O}(g)\)
- \(\ce{Zn}(s)+\ce{H2SO4}(aq)\rightarrow \ce{ZnSO4}(aq)+\ce{H2}(g)\)
- \(\ce{2K2S2O3}(s)+\ce{I2}(s)\rightarrow \ce{K2S4O6}(s)+\ce{2KI}(s)\)
- \(\ce{3Cu}(s)+\ce{8HNO3}(aq)\rightarrow\ce{3Cu(NO3)2}(aq)+\ce{2NO}(g)+\ce{4H2O}(l)\)
Q4.2.11
Jaza na usawazishe usawa wa asidi-msingi:
- Gesi ya HCl humenyuka na Ca imara (OH) 2 (s).
- Suluhisho la Sr (OH) 2 linaongezwa kwenye suluhisho la HNO 3.
S4.2.11
- \(\ce{2HCl}(g)+\ce{Ca(OH)2}(s)\rightarrow \ce{CaCl2}(s)+\ce{2H2O}(l)\);
- \(\ce{Sr(OH)2}(aq)+\ce{2HNO3}(aq)\rightarrow \ce{Sr(NO3)2}(aq)+\ce{2H2O}(l)\)
Q4.2.12
Jaza na usawazishe usawa wa asidi-msingi:
- Suluhisho la HClo 4 linaongezwa kwenye suluhisho la LiOH.
- Maji H 2 SO 4 humenyuka na NaOH.
- Ba (OH) 2 humenyuka na gesi ya HF.
Q4.2.13
Jaza na usawazishe athari zifuatazo za kupunguza oxidation, ambazo hutoa hali ya juu zaidi ya oxidation kwa atomi zilizooksidishwa.
- \(\ce{Al}(s)+\ce{F2}(g)\rightarrow\)
- \(\ce{Al}(s)+\ce{CuBr2}(aq)\rightarrow\)(uhamisho mmoja)
- \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \)
- \(\ce{Ca}(s)+\ce{H2O}(l)\rightarrow \)(bidhaa ni msingi wenye nguvu na gesi ya diatomic)
S4.2.13
- \(\ce{2Al}(s)+\ce{3F2}(g)\rightarrow \ce{2AlF3}(s)\);
- \(\ce{2Al}(s)+\ce{3CuBr2}(aq)\rightarrow \ce{3Cu}(s)+\ce{2AlBr3}(aq)\);
- \(\ce{P4}(s)+\ce{5O2}(g)\rightarrow \ce{P4O10}(s)\);\(\ce{Ca}(s)+\ce{2H2O}(l)\rightarrow \ce{Ca(OH)2}(aq)+\ce{H2}(g)\)
Q4.2.14
Jaza na usawazishe athari zifuatazo za kupunguza oxidation, ambazo hutoa hali ya juu zaidi ya oxidation kwa atomi zilizooksidishwa.
- \(\ce{K}(s)+\ce{H2O}(l)\rightarrow \)
- \(\ce{Ba}(s)+\ce{HBr}(aq)\rightarrow \)
- \(\ce{Sn}(s)+\ce{I2}(s)\rightarrow \)
Q4.2.15
Jaza na usawazishe equations kwa athari zifuatazo za asidi-msingi neutralization. Ikiwa maji hutumiwa kama kutengenezea, andika reactants na bidhaa kama ions yenye maji. Katika hali nyingine, kunaweza kuwa na jibu zaidi ya moja sahihi, kulingana na kiasi cha reactants kutumika.
- \(\ce{Mg(OH)2}(s)+\ce{HClO4}(aq)\rightarrow \)
- \(\ce{SO3}(g)+\ce{H2O}(l)\rightarrow \)(kudhani ziada ya maji na kwamba bidhaa hupasuka)
- \(\ce{SrO}(s)+\ce{H2SO4}(l)\rightarrow \)
S4.2.15
- \(\ce{Mg(OH)2}(s)+\ce{2HClO4}(aq)\rightarrow \ce{Mg^2+}(aq)+\ce{2ClO4-}(aq)+\ce{2H2O}(l)\);
- \(\ce{SO3}(g)+\ce{2H2O}(l)\rightarrow \ce{H3O+}(aq)+\ce{HSO4-}(aq)\), (suluhisho la H 2 SO 4);
- \(\ce{SrO}(s)+\ce{H2SO4}(l)\rightarrow \ce{SrSO4}(s)+\ce{H2O}\)
Q4.2.16
Wakati joto hadi 700—800 °C, almasi, ambayo ni kaboni safi, huoksidishwa na oksijeni ya angahewa. (Wao kuchoma!) Andika equation uwiano kwa mmenyuko huu.
Q4.2.17
Jeshi limejaribu lasers zinazozalisha mwanga mkali sana wakati fluorine inachanganya kwa ukali na hidrojeni. ni equation uwiano kwa mmenyuko huu ni nini?
S4.2.17
\(\ce{H2}(g)+\ce{F2}(g)\rightarrow \ce{2HF}(g)\)
Q4.2.18
Andika equations Masi, jumla ionic, na wavu ionic equations kwa athari zifuatazo:
- \(\ce{Ca(OH)2}(aq)+\ce{HC2H3O2}(aq)\rightarrow \)
- \(\ce{H3PO4}(aq)+\ce{CaCl2}(aq)\rightarrow \)
Q4.2.19
Kampuni ya Kemikali ya Maziwa Makuu hutoa bromini, Br 2, kutoka chumvi za bromidi kama vile NaBR, katika brine ya Arkansas kwa kutibu brine na gesi ya klorini. Andika usawa wa usawa kwa mmenyuko wa NaBR na Cl 2.
S4.2.19
\(\ce{2NaBr}(aq)+\ce{Cl2}(g)\rightarrow \ce{2NaCl}(aq)+\ce{Br2}(l)\)
Q4.2.20
Katika jaribio la kawaida katika maabara ya kemia ya jumla, chuma cha magnesiamu kinawaka hewa ili kuzalisha MgO. MgO ni imara nyeupe, lakini katika majaribio haya mara nyingi inaonekana kijivu, kutokana na kiasi kidogo cha Mg 3 N 2, kiwanja kilichoundwa kama baadhi ya magnesiamu humenyuka na nitrojeni. Andika equation uwiano kwa kila mmenyuko.
Q4.2.21
Hidroksidi ya lithiamu inaweza kutumika kunyonya dioksidi kaboni katika mazingira yaliyofungwa, kama vile spacecraft manned na submarines. Andika equation kwa mmenyuko unaohusisha 2 mol ya LiOH kwa 1 mol ya CO 2. (Kidokezo: Maji ni moja ya bidhaa.)
S4.2.21
\(\ce{2LiOH}(aq)+\ce{CO2}(g)\rightarrow \ce{Li2CO3}(aq)+\ce{H2O}(l)\)
Q4.2.22
Wakati mwingine calcium propionate huongezwa kwa mkate ili kuzuia uharibifu. Kiwanja hiki kinaweza kutayarishwa na mmenyuko wa carbonate ya kalsiamu, CaCO 3, na asidi ya propionic, C 2 H 5 CO 2 H, ambayo ina mali sawa na ile ya asidi ya asidi. Andika usawa wa usawa kwa ajili ya malezi ya propionate ya kalsiamu.
Q4.2.23
Jaza na usawa usawa wa athari zifuatazo, ambayo kila mmoja inaweza kutumika kuondoa sulfidi hidrojeni kutoka gesi asilia:
- \(\ce{Ca(OH)2}(s)+\ce{H2S}(g) \rightarrow\)
- \(\ce{Na2CO3}(aq)+\ce{H2S}(g)\rightarrow \)
S4.2.23
- \(\ce{Ca(OH)2}(s)+\ce{H2S}(g)\rightarrow \ce{CaS}(s)+\ce{2H2O}(l)\);
- \(\ce{Na2CO3}(aq)+\ce{H2S}(g)\rightarrow \ce{Na2S}(aq)+\ce{CO2}(g)+\ce{H2O}(l)\)
Q4.2.24
Sulfidi ya shaba (II) imeoksidishwa na oksijeni ya Masi ili kuzalisha trioxide ya sulfuri ya gesi na oksidi ya shaba imara (II). Bidhaa ya gesi kisha humenyuka na maji ya kioevu ili kuzalisha sulfate ya hidrojeni kioevu kama bidhaa pekee. Andika equations mbili ambazo zinawakilisha athari hizi.
Q4.2.25
Andika usawa wa kemikali kwa athari zilizotumiwa kuandaa kila moja ya misombo ifuatayo kutoka kwa nyenzo zilizopewa kuanzia. Katika hali nyingine, majibu ya ziada yanahitajika.
- nitrati imara ya amonia kutoka nitrojeni ya gesi ya Masi kupitia mchakato wa hatua mbili (kwanza kupunguza nitrojeni kwa amonia, halafu neutralize amonia na asidi sahihi)
- bromidi ya hidrojeni ya gesi kutoka kwa bromini ya Masi ya kioevu kupitia mmenyuko wa redox moja
- gesi H 2 S kutoka Zn imara na S kupitia mchakato wa hatua mbili (kwanza mmenyuko wa redox kati ya vifaa vya kuanzia, kisha mmenyuko wa bidhaa na asidi kali)
S4.2.25
- hatua ya 1:\(\ce{N2}(g)+\ce{3H2}(g)\rightarrow \ce{2NH3}(g)\), hatua ya 2:\(\ce{NH3}(g)+\ce{HNO3}(aq)\rightarrow \ce{NH4NO3}(aq)\rightarrow \ce{NH4NO3}(s)\ce{(after\: drying)}\);
- \(\ce{H2}(g)+\ce{Br2}(l)\rightarrow \ce{2HBr}(g)\);
- \(\ce{Zn}(s)+\ce{S}(s)\rightarrow \ce{ZnS}(s)\)na\(\ce{ZnS}(s)+\ce{2HCl}(aq)\rightarrow \ce{ZnCl2}(aq)+\ce{H2S}(g)\)
Q4.2.26
Calcium cyclamate Ca (C 6 H 11 NHSO 3) 2 ni sweetener bandia inayotumika katika nchi nyingi duniani kote lakini imepigwa marufuku nchini Marekani. Inaweza kujitakasa viwanda kwa kuwabadili kwa chumvi ya bariamu kwa njia ya mmenyuko wa asidi C 6 H 11 NHSO 3 H na carbonate ya bariamu, matibabu na asidi sulfuriki (sulfate ya bariamu haipatikani sana), na kisha neutralization na hidroksidi ya kalsiamu. Andika equations uwiano kwa athari hizi.
Q4.2.27
Jaza na usawazishe kila moja ya athari za nusu zifuatazo (hatua 2—5 katika njia ya nusu ya majibu):
- \(\ce{Sn^4+}(aq)\rightarrow \ce{Sn^2+}(aq)\)
- \(\ce{[Ag(NH3)2]+}(aq)\rightarrow \ce{Ag}(s)+\ce{NH3}(aq)\)
- \(\ce{Hg2Cl2}(s)\rightarrow \ce{Hg}(l)+\ce{Cl-}(aq)\)
- \(\ce{H2O}(l)\rightarrow \ce{O2}(g)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{IO3-}(aq)\rightarrow \ce{I2}(s)\)
- \(\ce{SO3^2-}(aq)\rightarrow \ce{SO4^2-}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{MnO4-}(aq)\rightarrow \ce{Mn^2+}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{Cl-}(aq)\rightarrow \ce{ClO3-}(aq)\ce{\:(in\: basic\: solution)}\)
S4.2.27
- \(\ce{Sn^4+}(aq)+\ce{2e-}\rightarrow \ce{Sn^2+}(aq)\),
- \(\ce{[Ag(NH3)2]+}(aq)+ \ce{e-} \rightarrow \ce{Ag}(s)+\ce{2NH3}(aq)\);
- \(\ce{Hg2Cl2}(s)+ \ce{2e-} \rightarrow \ce{2Hg}(l)+\ce{2Cl-}(aq)\);
- \(\ce{2H2O}(l)\rightarrow \ce{O2}(g)+\ce{4H+}(aq)+\ce{4e-}\);
- \(\ce{6H2O}(l)+\ce{2IO3-}(aq)+\ce{10e-}\rightarrow \ce{I2}(s)+\ce{12OH-}(aq)\);
- \(\ce{H2O}(l)+\ce{SO3^2-}(aq)\rightarrow \ce{SO4^2-}(aq)+\ce{2H+}(aq)+\ce{2e-}\);
- (g)\(\ce{8H+}(aq)+\ce{MnO4-}(aq)+\ce{5e-}\rightarrow \ce{Mn^2+}(aq)+\ce{4H2O}(l)\);
- (h)\(\ce{Cl-}(aq)+\ce{6OH-}(aq)\rightarrow \ce{ClO3-}(aq)+\ce{3H2O}(l)+\ce{6e-}\)
Q4.2.28
Jaza na usawazishe kila moja ya athari za nusu zifuatazo (hatua 2—5 katika njia ya nusu ya majibu):
- \(\ce{Cr^2+}(aq)\rightarrow \ce{Cr^3+}(aq)\)
- \(\ce{Hg}(l)+\ce{Br-}(aq)\rightarrow \ce{HgBr4^2-}(aq)\)
- \(\ce{ZnS}(s)\rightarrow \ce{Zn}(s)+\ce{S^2-}(aq)\)
- \(\ce{H2}(g)\rightarrow \ce{H2O}(l)\ce{\:(in\: basic\: solution)}\)
- \(\ce{H2}(g)\rightarrow \ce{H3O+}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{NO3-}(aq)\rightarrow \ce{HNO2}(aq)\ce{\:(in\: acidic\: solution)}\)
- \(\ce{MnO2}(s)\rightarrow \ce{MnO4-}(aq)\ce{\:(in\: basic\: solution)}\)
- \(\ce{Cl-}(aq)\rightarrow \ce{ClO3-}(aq)\ce{\:(in\: acidic\: solution)}\)
Q4.2.29
Mizani kila moja ya equations zifuatazo kulingana na njia ya nusu-mmenyuko:
- \(\ce{Sn^2+}(aq)+\ce{Cu^2+}(aq)\rightarrow \ce{Sn^4+}(aq)+\ce{Cu+}(aq)\)
- \(\ce{H2S}(g)+\ce{Hg2^2+}(aq)\rightarrow \ce{Hg}(l)+\ce{S}(s)\ce{\:(in\: acid)}\)
- \(\ce{CN-}(aq)+\ce{ClO2}(aq)\rightarrow \ce{CNO-}(aq)+\ce{Cl-}(aq)\ce{\:(in\: acid)}\)
- \(\ce{Fe^2+}(aq)+\ce{Ce^4+}(aq)\rightarrow \ce{Fe^3+}(aq)+\ce{Ce^3+}(aq)\)
- \(\ce{HBrO}(aq)\rightarrow \ce{Br-}(aq)+\ce{O2}(g)\ce{\:(in\: acid)}\)
S4.2.29
- \(\ce{Sn^2+}(aq)+\ce{2Cu^2+}(aq)\rightarrow \ce{Sn^4+}(aq)+\ce{2Cu+}(aq)\);
- \(\ce{H2S}(g)+\ce{Hg2^2+}(aq)+\ce{2H2O}(l)\rightarrow \ce{2Hg}(l)+\ce{S}(s)+\ce{2H3O+}(aq)\);
- \(\ce{5CN-}(aq)+\ce{2ClO2}(aq)+\ce{3H2O}(l)\rightarrow \ce{5CNO-}(aq)+\ce{2Cl-}(aq)+\ce{2H3O+}(aq)\);
- \(\ce{Fe^2+}(aq)+\ce{Ce^4+}(aq)\rightarrow \ce{Fe^3+}(aq)+\ce{Ce^3+}(aq)\);
- \(\ce{2HBrO}(aq)+\ce{2H2O}(l)\rightarrow \ce{2H3O+}(aq)+\ce{2Br-}(aq)+\ce{O2}(g)\)
Q4.2.30
Mizani kila moja ya equations zifuatazo kulingana na njia ya nusu-mmenyuko:
- \(\ce{Zn}(s)+\ce{NO3-}(aq)\rightarrow \ce{Zn^2+}(aq)+\ce{N2}(g)\ce{\:(in\: acid)}\)
- \(\ce{Zn}(s)+\ce{NO3-}(aq)\rightarrow \ce{Zn^2+}(aq)+\ce{NH3}(aq)\ce{\:(in\: base)}\)
- \(\ce{CuS}(s)+\ce{NO3-}(aq)\rightarrow \ce{Cu^2+}(aq)+\ce{S}(s)+\ce{NO}(g)\ce{\:(in\: acid)}\)
- \(\ce{NH3}(aq)+\ce{O2}(g)\rightarrow \ce{NO2}(g)\ce{\:(gas\: phase)}\)
- \(\ce{Cl2}(g)+\ce{OH-}(aq)\rightarrow \ce{Cl-}(aq)+\ce{ClO3-}(aq)\ce{\:(in\: base)}\)
- \(\ce{H2O2}(aq)+\ce{MnO4-}(aq)\rightarrow \ce{Mn^2+}(aq)+\ce{O2}(g)\ce{\:(in\: acid)}\)
- \(\ce{NO2}(g)\rightarrow \ce{NO3-}(aq)+\ce{NO2-}(aq)\ce{\:(in\: base)}\)
- \(\ce{Fe^3+}(aq)+\ce{I-}(aq)\rightarrow \ce{Fe^2+}(aq)+\ce{I2}(aq)\)
Q4.2.31
Mizani kila moja ya equations zifuatazo kulingana na njia ya nusu-mmenyuko:
- \(\ce{MnO4-}(aq)+\ce{NO2-}(aq)\rightarrow \ce{MnO2}(s)+\ce{NO3-}(aq)\ce{\:(in\: base)}\)
- \(\ce{MnO4^2-}(aq)\rightarrow \ce{MnO4-}(aq)+\ce{MnO2}(s)\ce{\:(in\: base)}\)
- \(\ce{Br2}(l)+\ce{SO2}(g)\rightarrow \ce{Br-}(aq)+\ce{SO4^2-}(aq)\ce{\:(in\: acid)}\)
S4.2.31
- \(\ce{2MnO4-}(aq)+\ce{3NO2-}(aq)+\ce{H2O}(l)\rightarrow \ce{2MnO2}(s)+\ce{3NO3-}(aq)+\ce{2OH-}(aq)\);
- \(\ce{3MnO4^2-}(aq)+\ce{2H2O}(l)\rightarrow \ce{2MnO4-}(aq)+\ce{4OH-}(aq)+\ce{MnO2}(s)\ce{\:(in\: base)}\);
- \(\ce{Br2}(l)+\ce{SO2}(g)+\ce{2H2O}(l)\rightarrow \ce{4H+}(aq)+\ce{2Br-}(aq)+\ce{SO4^2-}(aq)\)
4.3: Stoichiometry ya majibu
Q4.3.1
Andika usawa wa usawa, kisha ueleze hatua zinazohitajika ili kuamua habari zilizoombwa katika kila moja ya yafuatayo:
- Idadi ya moles na wingi wa klorini, Cl 2, inahitajika kuguswa na 10.0 g ya chuma cha sodiamu, Na, ili kuzalisha kloridi ya sodiamu, NaCl.
- Idadi ya moles na wingi wa oksijeni inayotengenezwa na kuharibika kwa 1.252 g ya oksidi ya zebaki (II).
- Idadi ya moles na wingi wa nitrati ya sodiamu, nano 3, inahitajika kuzalisha 128 g ya oksijeni. (NaNO 2 ni bidhaa nyingine.)
- Idadi ya moles na wingi wa dioksidi kaboni inayotengenezwa na mwako wa kilo 20.0 ya kaboni kwa ziada ya oksijeni.
- Idadi ya moles na wingi wa kaboni ya shaba (II) ilihitaji kuzalisha kilo 1.500 ya oksidi ya shaba (II). (CO 2 ni bidhaa nyingine.)

Q4.3.2
Kuamua idadi ya moles na wingi ombi kwa kila mmenyuko katika Zoezi.
S4.3.2
0.435 mol Na, 0.217 mol Cl 2, 15.4 g Cl 2; 0.005780 mol HgO, 2.890 × 10 —3 mol O 2, 9.248 × 10 -2 g O 2; 8.00 mol nano 3, 6.8 × 10 2 g Nano 3; 1665 mol CO 2, 73.3 kg CO 2 ; 18.86 mol CUO, 2.330 kg CuCO 3; 0.4580 mol C 2 H 4 rubles 2, 86.05 g C 2 H 4 rubles 2
Q4.3.3
Andika usawa wa usawa, kisha ueleze hatua zinazohitajika ili kuamua habari zilizoombwa katika kila moja ya yafuatayo:
- Idadi ya moles na wingi wa Mg inahitajika kuguswa na 5.00 g ya HCl na kuzalisha MgCl 2 na H 2.
- Idadi ya moles na wingi wa oksijeni inayotengenezwa na kuharibika kwa 1.252 g ya oksidi ya fedha (I).
- Idadi ya moles na wingi wa carbonate ya magnesiamu, MgCo 3, inahitajika kuzalisha 283 g ya dioksidi kaboni. (MgO ni bidhaa nyingine.)
- Idadi ya moles na wingi wa maji uliofanywa na mwako wa kilo 20.0 ya acetylene, C 2 H 2, kwa ziada ya oksijeni.
- Idadi ya moles na wingi wa peroxide ya bariamu, BaO 2, inahitajika kuzalisha kilo 2.500 ya oksidi ya bariamu, BaO (O 2 ni bidhaa nyingine.)

Q4.3.4
Kuamua idadi ya moles na wingi ombi kwa kila mmenyuko katika Zoezi.
S4.3.4
0.0686 ml Mg, 1.67 g Mg; 2.701 × 10 -3 mL O 2, 0.08644 g O 2; 6.43 mL MgCo 3, 542 g MgCo 3 713 mL H 2 O, 12.8 kg H 2 O; 16.31 mol BaO 2, 2762 g BaO 2; 0.207 mL C 2 H 4 , 5.81 g C 2 H 4
Q4.3.5
H 2 huzalishwa na mmenyuko wa 118.5 ml ya ufumbuzi wa 0.87755-M wa H 3 PO 4 kulingana na equation ifuatayo:\(\ce{2Cr + 2H3PO4 \rightarrow 3H2 + 2CrPO4}\).
- Outline the steps necessary to determine the number of moles and mass of H2.
- Perform the calculations outlined.
S4.3.5
a.)
- Convert mL to L
- Multiply L by the molarity to determine moles of H3PO4
- Convert moles of H3PO4 to moles of H2
- Multiply moles of H2 by the molar mass of H2 to get the answer in grams
b.)
1. \(118.5\: mL\times \dfrac{1\: L}{1000\: mL} = 0.1185\: L\)
2. \(0.1185\: L \times \dfrac{0.8775\: moles\: \ce{H3PO4}}{1\: L} = 0.1040\: moles\: \ce{H3PO4}\)
3. \(0.1040\: moles\: \ce{H3PO4} \times \dfrac{3\: moles\:\ce{H_2}}{2\: moles\: \ce{H3PO4}} = 0.1560\: moles\: \ce{H2}\)
4. \(0.1560\: moles\: \ce{H2} \times \dfrac{2.02 g}{1\: mole} = 0.3151g\: \ce{H2}\)
Q4.3.6
Gallium chloride is formed by the reaction of 2.6 L of a 1.44 M solution of HCl according to the following equation: \(\ce{2Ga + 6HCl \rightarrow 2GaCl3 + 3H2}\).
- Outline the steps necessary to determine the number of moles and mass of gallium chloride.
- Perform the calculations outlined.
S4.3.6
\(\mathrm{volume\: HCl\: solution \rightarrow mol\: HCl \rightarrow mol\: GaCl_3}\); 1.25 mol GaCl3, 2.2 × 102 g GaCl3
Q4.3.7
I2 is produced by the reaction of 0.4235 mol of CuCl2 according to the following equation: \(\ce{2CuCl2 + 4KI \rightarrow 2CuI + 4KCl + I2}\).
- How many molecules of I2 are produced?
- What mass of I2 is produced?
Q4.3.8
Silver is often extracted from ores as K[Ag(CN)2] and then recovered by the reaction
\(\ce{2K[Ag(CN)2]}(aq)+\ce{Zn}(s)\rightarrow \ce{2Ag}(s)+\ce{Zn(CN)2}(aq)+\ce{2KCN}(aq)\)
- How many molecules of Zn(CN)2 are produced by the reaction of 35.27 g of K[Ag(CN)2]?
- What mass of Zn(CN)2 is produced?
S4.3.8
5.337 × 1022 molecules; 10.41 g Zn(CN)2
Q4.3.9
What mass of silver oxide, Ag2O, is required to produce 25.0 g of silver sulfadiazine, AgC10H9N4SO2, from the reaction of silver oxide and sulfadiazine?
\(\ce{2C10H10N4SO2 + Ag2O \rightarrow 2AgC10H9N4SO2 + H2O}\)
Q4.3.10
Carborundum is silicon carbide, SiC, a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, SiO2, with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. Write the balanced equation for the reaction, and calculate how much SiO2 is required to produce 3.00 kg of SiC.
S4.3.10
\(\ce{SiO2 + 3C \rightarrow SiC + 2CO}\), 4.50 kg SiO2
Q4.3.11
Automotive air bags inflate when a sample of sodium azide, NaN3, is very rapidly decomposed.
\(\ce{2NaN3}(s) \rightarrow \ce{2Na}(s) + \ce{3N2}(g)\)
What mass of sodium azide is required to produce 2.6 ft3 (73.6 L) of nitrogen gas with a density of 1.25 g/L?
S4.3.11
142g NaN3
Q4.3.12
Urea, CO(NH2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. What is the maximum mass of urea that can be manufactured from the CO2 produced by combustion of 1.00×103 kg of carbon followed by the reaction?
\[\ce{CO2}(g)+\ce{2NH3}(g)\rightarrow \ce{CO(NH2)2}(s)+\ce{H2O}(l)\]
S4.3.12
5.00 × 103 kg
Q4.3.13
In an accident, a solution containing 2.5 kg of nitric acid was spilled. Two kilograms of Na2CO3 was quickly spread on the area and CO2 was released by the reaction. Was sufficient Na2CO3 used to neutralize all of the acid?
Q4.3.14
A compact car gets 37.5 miles per gallon on the highway. If gasoline contains 84.2% carbon by mass and has a density of 0.8205 g/mL, determine the mass of carbon dioxide produced during a 500-mile trip (3.785 liters per gallon).
S4.3.14
1.28 × 105 g CO2
Q4.3.15
What volume of a 0.750 M solution of hydrochloric acid, a solution of HCl, can be prepared from the HCl produced by the reaction of 25.0 g of NaCl with an excess of sulfuric acid?
\[\ce{NaCl}(s)+\ce{H2SO4}(l)\rightarrow \ce{HCl}(g)+\ce{NaHSO4}(s)\]
Q4.3.16
What volume of a 0.2089 M KI solution contains enough KI to react exactly with the Cu(NO3)2 in 43.88 mL of a 0.3842 M solution of Cu(NO3)2?
\[\ce{2Cu(NO3)2 + 4KI \rightarrow 2CuI + I2 + 4KNO3}\]
S4.3.16
161.40 mL KI solution
Q4.3.17
A mordant is a substance that combines with a dye to produce a stable fixed color in a dyed fabric. Calcium acetate is used as a mordant. It is prepared by the reaction of acetic acid with calcium hydroxide.
\[\ce{2CH3CO2H + Ca(OH)2 \rightarrow Ca(CH3CO2)2 + 2H2O}\]
What mass of Ca(OH)2 is required to react with the acetic acid in 25.0 mL of a solution having a density of 1.065 g/mL and containing 58.0% acetic acid by mass?
Q4.3.18
The toxic pigment called white lead, Pb3(OH)2(CO3)2, has been replaced in white paints by rutile, TiO2. How much rutile (g) can be prepared from 379 g of an ore that contains 88.3% ilmenite (FeTiO3) by mass?
\[\ce{2FeTiO3 + 4HCl + Cl2 \rightarrow 2FeCl3 + 2TiO2 + 2H2O}\]
S4.3.18
176 g TiO2
4.4: Reaction Yields
Q4.4.1
The following quantities are placed in a container: 1.5 × 1024 atoms of hydrogen, 1.0 mol of sulfur, and 88.0 g of diatomic oxygen.
- What is the total mass in grams for the collection of all three elements?
- What is the total number of moles of atoms for the three elements?
- If the mixture of the three elements formed a compound with molecules that contain two hydrogen atoms, one sulfur atom, and four oxygen atoms, which substance is consumed first?
- How many atoms of each remaining element would remain unreacted in the change described in ?
Q4.4.2
What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g of diatomic chlorine?
S4.4.2
The limiting reactant is Cl2.
Q4.4.3
Which of the postulates of Dalton's atomic theory explains why we can calculate a theoretical yield for a chemical reaction?
Q4.4.4
A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g. What was his percent yield?
S4.4.4
\(\mathrm{Percent\: yield = 31\%}\)
Q4.4.5
A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction?
\[\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(s)\]
Q4.4.6
Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl2F2 from 32.9 g of CCl4. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield.
S4.4.6
\(\ce{g\: CCl4\rightarrow mol\: CCl4\rightarrow mol\: CCl2F2 \rightarrow g\: CCl2F2}, \mathrm{\:percent\: yield=48.3\%}\)
Q4.4.7
Citric acid, C6H8O7, a component of jams, jellies, and fruity soft drinks, is prepared industrially via fermentation of sucrose by the mold Aspergillus niger. The equation representing this reaction is
\[\ce{C12H22O11 + H2O + 3O2 \rightarrow 2C6H8O7 + 4H2O}\]
What mass of citric acid is produced from exactly 1 metric ton (1.000 × 103 kg) of sucrose if the yield is 92.30%?
Q4.4.8
Toluene, C6H5CH3, is oxidized by air under carefully controlled conditions to benzoic acid, C6H5CO2H, which is used to prepare the food preservative sodium benzoate, C6H5CO2Na. What is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?
\[\ce{2C6H5CH3 + 3O2 \rightarrow 2C6H5CO2H + 2H2O}\]
S4.4.8
\(\mathrm{percent\: yield=91.3\%}\)
Q4.4.9
In a laboratory experiment, the reaction of 3.0 mol of H2 with 2.0 mol of I2 produced 1.0 mol of HI. Determine the theoretical yield in grams and the percent yield for this reaction.
Q4.4.10
Outline the steps needed to solve the following problem, then do the calculations. Ether, (C2H5)2O, which was originally used as an anesthetic but has been replaced by safer and more effective medications, is prepared by the reaction of ethanol with sulfuric acid.
2C2H5OH + H2SO4 ⟶ (C2H5)2 + H2SO4·H2O
Q4.4.11
What is the percent yield of ether if 1.17 L (d = 0.7134 g/mL) is isolated from the reaction of 1.500 L of C2H5OH (d = 0.7894 g/mL)?
S4.4.11
Convert mass of ethanol to moles of ethanol; relate the moles of ethanol to the moles of ether produced using the stoichiometry of the balanced equation. Convert moles of ether to grams; divide the actual grams of ether (determined through the density) by the theoretical mass to determine the percent yield; 87.6%
Q4.4.12
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, C3H8, is burned with 75.0 g of oxygen.
\[\mathrm{percent\: yield=\dfrac{0.8347\:\cancel{g}}{0.9525\:\cancel{g}}\times 100\%=87.6\%}\]
Determine the limiting reactant.
Q4.4.13
Outline the steps needed to determine the limiting reactant when 0.50 g of Cr and 0.75 g of H3PO4 react according to the following chemical equation?
\[\ce{2Cr + 2H3PO4 \rightarrow 2CrPO4 + 3H2}\]
Determine the limiting reactant.
S4.4.13
The conversion needed is \(\ce{mol\: Cr \rightarrow mol\: H2PO4}\). Then compare the amount of Cr to the amount of acid present. Cr is the limiting reactant.
Q4.4.14
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium nitride, a component of advanced batteries, according to the following unbalanced equation?
\[\ce{Li + N2 \rightarrow Li3N}\]
S4.4.14
\[\ce{6Li} + \ce{N2} \rightarrow \: \ce{2Li3N}\]
\[1.50g\: \ce{Li} \times \dfrac{1\: mole\: \ce{Li}}{6.94g\: \ce{Li}} \times\dfrac{2\: mole\: \ce{Li3N}}{6\:mole\: \ce{Li}} = 0.0720\: moles\: \ce{Li3N}\]
\[1.50g\: \ce{N2} \times \dfrac{1\: mole\: \ce{N2}}{28.02g\: \ce{N2}} \times\dfrac{2\: mole\: \ce{Li3N}}{1\:mole\: \ce{N2}} = 0.107\: moles\: \ce{Li3N}\]
\(\ce{Li}\) is the limiting reactant
Q4.4.15
Uranium can be isolated from its ores by dissolving it as UO2(NO3)2, then separating it as solid UO2(C2O4)·3H2O. Addition of 0.4031 g of sodium oxalate, Na2C2O4, to a solution containing 1.481 g of uranyl nitrate, UO2(NO2)2, yields 1.073 g of solid UO2(C2O4)·3H2O.
\[\ce{Na2C2O4 + UO2(NO3)2 + 3H2O ⟶ UO2(C2O4)·3H2O + 2NaNO3}\]
Determine the limiting reactant and the percent yield of this reaction.
S4.4.15
Na2C2O4 is the limiting reactant. percent yield = 86.6%
Q4.4.16
How many molecules of C2H4Cl2 can be prepared from 15 C2H4 molecules and 8 Cl2 molecules?
Q4.4.17
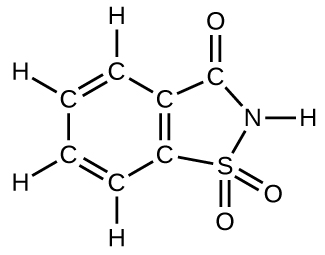
How many molecules of the sweetener saccharin can be prepared from 30 C atoms, 25 H atoms, 12 O atoms, 8 S atoms, and 14 N atoms?
S4.4.17
Only four molecules can be made.
Q4.4.18
The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen.
- What is the limiting reactant when 0.200 mol of P4 and 0.200 mol of O2 react according to \[\ce{P4 + 5O2 \rightarrow P4O10}\]
- Calculate the percent yield if 10.0 g of P4O10 is isolated from the reaction.
Q4.4.19
Would you agree to buy 1 trillion (1,000,000,000,000) gold atoms for $5? Explain why or why not. Find the current price of gold at http://money.cnn.com/data/commodities/ \(\mathrm{(1\: troy\: ounce=31.1\: g)}\)
S4.4.19
This amount cannot be weighted by ordinary balances and is worthless.
4.5: Quantitative Chemical Analysis
Q4.5.1
What volume of 0.0105-M HBr solution is be required to titrate 125 mL of a 0.0100-M Ca(OH)2 solution?
\[\ce{Ca(OH)2}(aq)+\ce{2HBr}(aq) \rightarrow \ce{CaBr2}(aq)+\ce{2H2O}(l)\]
Q4.5.2
Titration of a 20.0-mL sample of acid rain required 1.7 mL of 0.0811 M NaOH to reach the end point. If we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration of sulfuric acid in this sample of rain?
S4.5.2
3.4 × 10−3 M H2SO4
Q4.5.3
What is the concentration of NaCl in a solution if titration of 15.00 mL of the solution with 0.2503 M AgNO3 requires 20.22 mL of the AgNO3 solution to reach the end point?
\[\ce{AgNO3}(aq)+\ce{NaCl}(aq)\rightarrow \ce{AgCl}(s)+\ce{NaNO3}(aq)\]
Q4.5.4
In a common medical laboratory determination of the concentration of free chloride ion in blood serum, a serum sample is titrated with a Hg(NO3)2 solution.
\[\ce{2Cl-}(aq)+\ce{Hg(NO3)2}(aq)\rightarrow \ce{2NO3-}(aq)+\ce{HgCl2}(s)\]
What is the Cl− concentration in a 0.25-mL sample of normal serum that requires 1.46 mL of 5.25 × 10−4 M Hg(NO3)2(aq) to reach the end point?
S4.5.4
9.6 × 10−3 M Cl−
Q4.5.5
Potatoes can be peeled commercially by soaking them in a 3-M to 6-M solution of sodium hydroxide, then removing the loosened skins by spraying them with water. Does a sodium hydroxide solution have a suitable concentration if titration of 12.00 mL of the solution requires 30.6 mL of 1.65 M HCI to reach the end point?
Q4.5.6
A sample of gallium bromide, GaBr2, weighing 0.165 g was dissolved in water and treated with silver nitrate, AgNO3, resulting in the precipitation of 0.299 g AgBr. Use these data to compute the %Ga (by mass) GaBr2.
S4.5.6
22.4%
Q4.5.7
The principal component of mothballs is naphthalene, a compound with a molecular mass of about 130 amu, containing only carbon and hydrogen. A 3.000-mg sample of naphthalene burns to give 10.3 mg of CO2. Determine its empirical and molecular formulas.
Q4.5.8
A 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu, burns spontaneously when exposed to air, producing 0.063 g of B2O3. What are the empirical and molecular formulas of the compound.
S4.5.8
The empirical formula is BH3. The molecular formula is B2H6.
Q4.5.9
Sodium bicarbonate (baking soda), NaHCO3, can be purified by dissolving it in hot water (60 °C), filtering to remove insoluble impurities, cooling to 0 °C to precipitate solid NaHCO3, and then filtering to remove the solid, leaving soluble impurities in solution. Any NaHCO3 that remains in solution is not recovered. The solubility of NaHCO3 in hot water of 60 °C is 164 g L. Its solubility in cold water of 0 °C is 69 g/L. What is the percent yield of NaHCO3 when it is purified by this method?
Q4.5.10
What volume of 0.600 M HCl is required to react completely with 2.50 g of sodium hydrogen carbonate?
\[\ce{NaHCO3}(aq)+\ce{HCl}(aq)\rightarrow \ce{NaCl}(aq)+\ce{CO2}(g)+\ce{H2O}(l)\]
S4.5.10
49.6 mL
Q4.5.11
What volume of 0.08892 M HNO3 is required to react completely with 0.2352 g of potassium hydrogen phosphate?
\[\ce{2HNO3}(aq)+\ce{K2HPO4}(aq)\rightarrow \ce{H2PO4}(aq)+\ce{2KNO3}(aq)\]
Q4.5.12
What volume of a 0.3300-M solution of sodium hydroxide would be required to titrate 15.00 mL of 0.1500 M oxalic acid?
\[\ce{C2O4H2}(aq)+\ce{2NaOH}(aq)\rightarrow \ce{Na2C2O4}(aq)+\ce{2H2O}(l)\]
S4.5.12
13.64 mL
Q4.5.13
What volume of a 0.00945-M solution of potassium hydroxide would be required to titrate 50.00 mL of a sample of acid rain with a H2SO4 concentration of 1.23 × 10−4 M.
\[\ce{H2SO4}(aq)+\ce{2KOH}(aq)\rightarrow \ce{K2SO4}(aq)+\ce{2H2O}(l)\]
S4.5.13
1.30 mL
Q4.5.14
A sample of solid calcium hydroxide, Ca(OH)2, is allowed to stand in water until a saturated solution is formed. A titration of 75.00 mL of this solution with 5.00 × 10−2 M HCl requires 36.6 mL of the acid to reach the end point.
\[\ce{Ca(OH)2}(aq)+\ce{2HCl}(aq)\rightarrow \ce{CaCl2}(aq)+\ce{2H2O}(l)\]
What is the molarity?
S4.5.14
1.22 M
Q4.5.15
What mass of Ca(OH)2 will react with 25.0 g of propionic acid to form the preservative calcium propionate according to the equation?
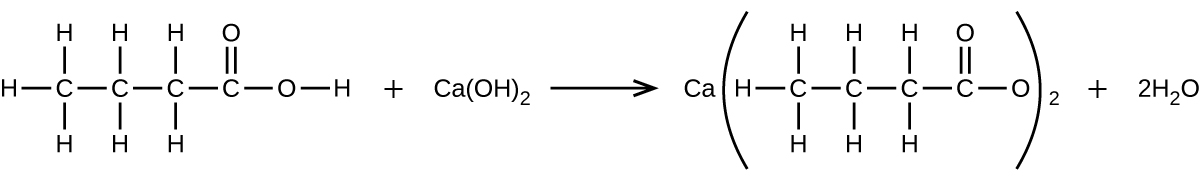
Q4.5.16
How many milliliters of a 0.1500-M solution of KOH will be required to titrate 40.00 mL of a 0.0656-M solution of H3PO4?
\[\ce{H3PO4}(aq)+\ce{2KOH}(aq)\rightarrow \ce{K2HPO4}(aq)+\ce{2H2O}(l)\]
S4.5.16
34.99 mL KOH
Q4.5.17
Potassium acid phthalate, KHC6H4O4, or KHP, is used in many laboratories, including general chemistry laboratories, to standardize solutions of base. KHP is one of only a few stable solid acids that can be dried by warming and weighed. A 0.3420-g sample of KHC6H4O4 reacts with 35.73 mL of a NaOH solution in a titration. What is the molar concentration of the NaOH?
\[\ce{KHC6H4O4}(aq)+\ce{NaOH}(aq)\rightarrow \ce{KNaC6H4O4}(aq)+\ce{H2O}(aq)\]
Q4.5.18
The reaction of WCl6 with Al at ~400 °C gives black crystals of a compound containing only tungsten and chlorine. A sample of this compound, when reduced with hydrogen, gives 0.2232 g of tungsten metal and hydrogen chloride, which is absorbed in water. Titration of the hydrochloric acid thus produced requires 46.2 mL of 0.1051 M NaOH to reach the end point. What is the empirical formula of the black tungsten chloride?
S4.5.19
The empirical formula is WCl4.


