3.6 : Capacités thermiques d'un gaz idéal
- Page ID
- 190992
À la fin de cette section, vous serez en mesure de :
- Définir la capacité thermique d'un gaz idéal pour un procédé spécifique
- Calculez la chaleur spécifique d'un gaz idéal pour un processus isobare ou isochore
- Expliquer la différence entre les capacités calorifiques d'un gaz idéal et d'un gaz réel
- Estimer la variation de la chaleur spécifique d'un gaz sur des plages de températures
Nous avons déjà découvert la chaleur spécifique et la capacité calorifique molaire ; toutefois, nous n'avons pas envisagé de processus par lequel de la chaleur est ajoutée. C'est ce que nous faisons dans cette section. Nous examinons d'abord un processus dans lequel le système a un volume constant, puis nous le comparons à un système à pression constante et montrons comment leurs chaleurs spécifiques sont liées.
Commençons par regarder la figure\(\PageIndex{1}\), qui montre deux récipients A et B, contenant chacun 1 mol du même type de gaz idéal à une température T et un volume V. La seule différence entre les deux cuves est que le piston situé au sommet de A est fixe, tandis que celui situé au sommet de B est libre de se déplacer à l'encontre d'une pression extérieure constante p. Nous examinons maintenant ce qui se passe lorsque la température du gaz dans chaque récipient augmente lentement\(T + dT\) avec l'ajout de chaleur.
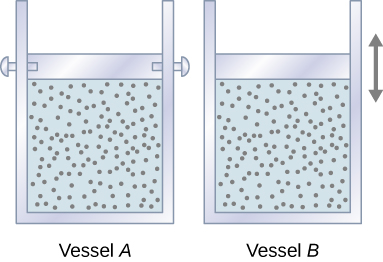
Since the piston of vessel A is fixed, the volume of the enclosed gas does not change. Consequently, the gas does no work, and we have from the first law
\[dE_{int} = dQ - dW = dQ.\]
We represent the fact that the heat is exchanged at constant volume by writing
\[dQ = C_VndT,\] where \(C_V\) is the molar heat capacity at constant volume of the gas. In addition, since \(dE_{int} = dQ\) for this particular process,
\[dE_{int} = C_VndT. \label{3.9}\]
We obtained this equation assuming the volume of the gas was fixed. However, internal energy is a state function that depends on only the temperature of an ideal gas. Therefore, \(dE_{int} = C_VndT\) gives the change in internal energy of an ideal gas for any process involving a temperature change dT.
When the gas in vessel B is heated, it expands against the movable piston and does work \(dW = pdV\). In this case, the heat is added at constant pressure, and we write \[dQ = C_{p}ndT,\] where \(C_p\) is the molar heat capacity at constant pressure of the gas. Furthermore, since the ideal gas expands against a constant pressure,
\[d(pV) = d(RnT)\] becomes \[pdV = RndT.\]
Finally, inserting the expressions for dQ and pdV into the first law, we obtain
\[dE_{int} = dQ - pdV = (C_{p}n - Rn)dT.\]
We have found \(dE_{int}\) for both an isochoric and an isobaric process. Because the internal energy of an ideal gas depends only on the temperature, \(dE_{int}\) must be the same for both processes. Thus,
\[C_{V}ndT = (C_{p}n - Rn)dT,\]
and
\[C_p = C_V + R. \label{eq50}\]
The derivation of Equation \ref{eq50} was based only on the ideal gas law. Consequently, this relationship is approximately valid for all dilute gases, whether monatomic like He, diatomic like \(O_2\), or polyatomic like \(CO_2\) or \(NH_3\).
In the preceding chapter, we found the molar heat capacity of an ideal gas under constant volume to be
\[C_V = \dfrac{d}{2}R,\]
where d is the number of degrees of freedom of a molecule in the system. Table \(\PageIndex{1}\) shows the molar heat capacities of some dilute ideal gases at room temperature. The heat capacities of real gases are somewhat higher than those predicted by the expressions of \(C_V\) and \(C_p\) given in Equation \ref{eq50}. This indicates that vibrational motion in polyatomic molecules is significant, even at room temperature. Nevertheless, the difference in the molar heat capacities, \(C_p - C_V\), is very close to R, even for the polyatomic gases.
| \(C_p\) | \(C_V\) | \(C_p - C_V\) | ||
|---|---|---|---|---|
| Type of Molecule | Gas | \(C_p\)" style="text-align:center;" class="lt-phys-4362">(J/mol K) | \(C_V\)" style="text-align:center;" class="lt-phys-4362">(J/mol K) | \(C_p - C_V\)" style="text-align:center;" class="lt-phys-4362">(J/mol K) |
| Monatomic | Ideal | \(C_p\)" style="text-align:center;" class="lt-phys-4362">\(\frac{5}{2}R = 20.79\) | \(C_V\)" style="text-align:center;" class="lt-phys-4362">\(\frac{3}{2}R = 12.47\) | \(C_p - C_V\)" style="text-align:center;" class="lt-phys-4362">\(R = 8.31\) |
| Diatomic | Ideal | \(C_p\)" style="text-align:center;" class="lt-phys-4362">\(\frac{7}{2}R = 29.10\) | \(C_V\)" style="text-align:center;" class="lt-phys-4362">\(\frac{5}{2}R = 20.79\) | \(C_p - C_V\)" style="text-align:center;" class="lt-phys-4362">\(R = 8.31\) |
| Polyatomic | Ideal | \(C_p\)" style="text-align:center;" class="lt-phys-4362">\(4R = 33.26\) | \(C_V\)" style="text-align:center;" class="lt-phys-4362">\(3R = 24.04\) | \(C_p - C_V\)" style="text-align:center;" class="lt-phys-4362">\(R = 8.31\) |
Glossary
- molar heat capacity at constant pressure
- quantifies the ratio of the amount of heat added removed to the temperature while measuring at constant pressure
- molar heat capacity at constant volume
- quantifies the ratio of the amount of heat added removed to the temperature while measuring at constant volume


