12.1: Ubaguzi
- Page ID
- 182519
Ujuzi wa Kuendeleza
- Tofautisha kati ya michakato ya pekee na isiyo ya kawaida
- Eleza usambazaji wa suala na nishati inayoambatana na michakato fulani ya hiari
Katika sehemu hii, fikiria tofauti kati ya aina mbili za mabadiliko katika mfumo: Yale yanayotokea kwa hiari na yale yanayotokea kwa nguvu. Kwa kufanya hivyo, tutaweza kupata ufahamu kuhusu kwa nini baadhi ya mifumo ni kawaida kutega mabadiliko katika mwelekeo mmoja chini ya hali fulani na jinsi ya haraka au polepole kwamba mabadiliko ya asili yanaendelea. Tutaweza pia kupata ufahamu katika jinsi spontaneity ya mchakato huathiri usambazaji wa nishati na suala ndani ya mfumo.
Michakato ya hiari na isiyo ya kawaida
Michakato ina tabia ya asili ya kutokea katika mwelekeo mmoja chini ya seti ya masharti. Maji yatapita kati yake kwa kawaida, lakini mtiririko wa kupanda unahitaji kuingilia nje kama vile matumizi ya pampu. Iron inayoonekana kwa anga ya dunia itapungua, lakini kutu haibadilishwa kuwa chuma bila matibabu ya kemikali ya makusudi. Mchakato wa pekee ni moja ambayo hutokea kwa kawaida chini ya hali fulani. Mchakato usio wa kawaida, kwa upande mwingine, hautafanyika isipokuwa “unaendeshwa” na pembejeo ya nishati ya kuendelea kutoka chanzo cha nje. Mchakato ambao ni wa pekee katika mwelekeo mmoja chini ya seti fulani ya masharti ni isiyo ya kawaida katika mwelekeo wa nyuma. Kwa joto la kawaida na shinikizo la kawaida la anga, kwa mfano, barafu litayeyuka kwa hiari, lakini maji hayatafungia kwa hiari.
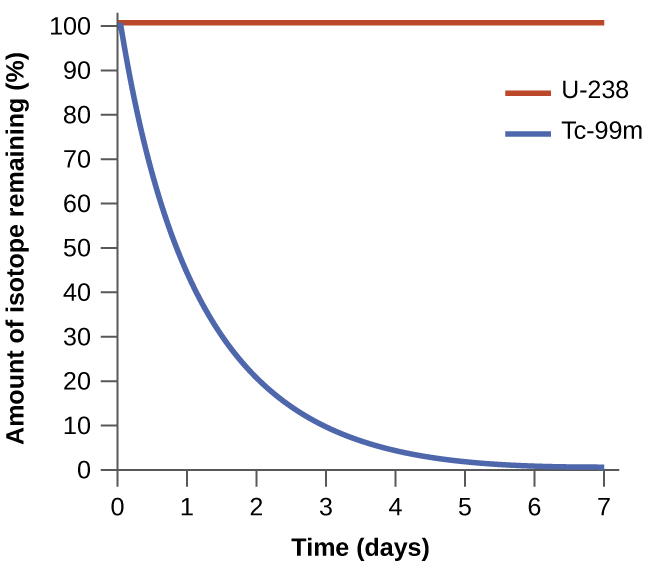
Uwezeshaji wa mchakato hauhusiani na kasi ya mchakato. Mabadiliko ya hiari yanaweza kuwa ya haraka sana kwamba kimsingi ni instantaneous au hivyo polepole kwamba haiwezi kuzingatiwa katika kipindi chochote cha vitendo. Ili kuonyesha dhana hii, fikiria kuoza kwa isotopu za mionzi, mada ya kutibiwa vizuri zaidi katika sura ya kemia ya nyuklia. Kuoza kwa mionzi ni kwa ufafanuzi mchakato wa hiari ambapo viini vya isotopu zisizo na uhakika hutoa mionzi kama zinabadilishwa kuwa nuclei imara zaidi. Michakato yote ya kuoza hutokea kwa hiari, lakini viwango ambavyo isotopu tofauti huoza hutofautiana sana. Technetium-99m ni radioisotopu maarufu kwa masomo ya upigaji picha za kimatibabu ambayo inakabiliwa na kuoza kwa haraka kiasi na inaonyesha nusu ya maisha ya saa sita. Uranium-238 ni isotopu nyingi zaidi ya uranium, na kuoza kwake hutokea polepole zaidi, kuonyesha nusu ya maisha ya zaidi ya miaka bilioni nne (Kielelezo\(\PageIndex{1}\)).
Kama mfano mwingine, fikiria uongofu wa almasi ndani ya grafiti (Kielelezo\(\PageIndex{2}\)).
\[\ce{C}_{(s,\textrm{ diamond})}⟶\ce{C}_{(s,\textrm{ graphite})} \label{Eq1}\]
Mchoro wa awamu ya kaboni unaonyesha kuwa grafiti ni fomu imara ya kipengele hiki chini ya shinikizo la anga la kawaida, wakati almasi ni allotrope imara katika shinikizo la juu sana, kama vile wale waliopo wakati wa malezi yake ya kijiolojia. Thermodynamic mahesabu ya aina ilivyoelezwa katika sehemu ya mwisho ya sura hii zinaonyesha kuwa uongofu wa almasi kwa grafiti katika shinikizo iliyoko hutokea kuwaka, lakini almasi ni aliona kuwepo, na kuendelea, chini ya masharti haya. Ingawa mchakato huo ni wa pekee chini ya hali ya kawaida, kiwango chake ni polepole sana, na hivyo kwa madhumuni yote ya vitendo almasi ni kweli “milele.” Hali kama hizi zinasisitiza tofauti muhimu kati ya mambo ya thermodynamic na kinetic ya mchakato. Katika kesi hii, almasi inasemekana kuwa thermodynamically imara lakini kinetically imara chini ya hali ya kawaida.
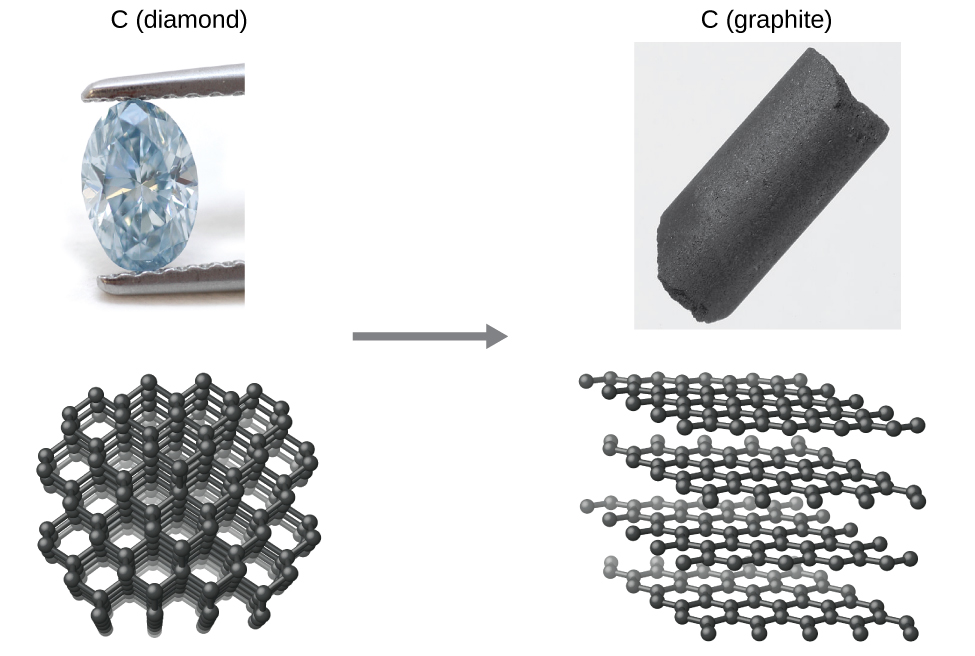
Kielelezo\(\PageIndex{2}\):The conversion of carbon from the diamond allotrope to the graphite allotrope is spontaneous at ambient pressure, but its rate is immeasurably slow at low to moderate temperatures. This process is known as graphitization, and its rate can be increased to easily measurable values at temperatures in the 1000–2000 K range. (credit "diamond" photo: modification of work by "Fancy Diamonds"/Flickr; credit "graphite" photo: modificaton of work by images-of-elements.com/carbon.php)
Dispersal of Matter and Energy
As we extend our discussion of thermodynamic concepts toward the objective of predicting spontaneity, consider now an isolated system consisting of two flasks connected with a closed valve. Initially there is an ideal gas on the left and a vacuum on the right (Figure \(\PageIndex{3}\)). When the valve is opened, the gas spontaneously expands to fill both flasks. Recalling the definition of pressure-volume work from the chapter on thermochemistry, note that no work has been done because the pressure in a vacuum is zero.
\[w=−PΔV=0 \;\;\; \mathrm{(P=0\: in\: a\: vaccum)} \label{Eq2}\]
Note as well that since the system is isolated, no heat has been exchanged with the surroundings (q = 0). The first law of thermodynamics confirms that there has been no change in the system’s internal energy as a result of this process.
\[ΔU=q+w=0+0=0 \label{Eq3}\]
The spontaneity of this process is therefore not a consequence of any change in energy that accompanies the process. Instead, the driving force appears to be related to the greater, more uniform dispersal of matter that results when the gas is allowed to expand. Initially, the system was comprised of one flask containing matter and another flask containing nothing. After the spontaneous process took place, the matter was distributed both more widely (occupying twice its original volume) and more uniformly (present in equal amounts in each flask).
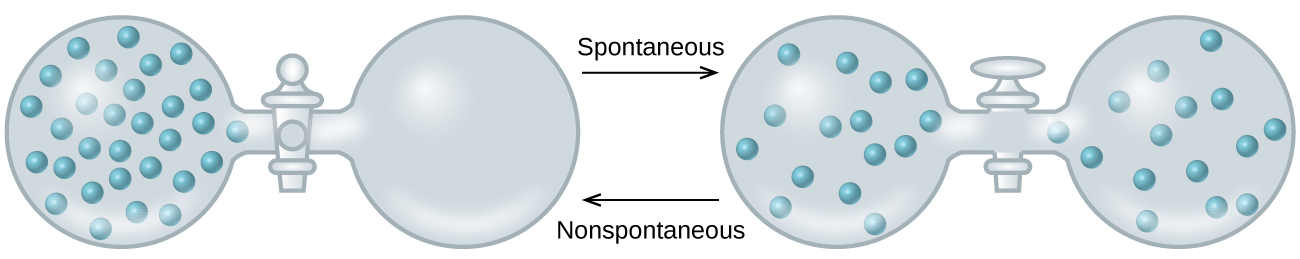
Kielelezo\(\PageIndex{3}\):An isolated system consists of an ideal gas in one flask that is connected by a closed valve to a second flask containing a vacuum. Once the valve is opened, the gas spontaneously becomes evenly distributed between the flasks.
Now consider two objects at different temperatures: object X at temperature TX and object Y at temperature TY, with TX > TY (Figure \(\PageIndex{4}\)). When these objects come into contact, heat spontaneously flows from the hotter object (X) to the colder one (Y). This corresponds to a loss of thermal energy by X and a gain of thermal energy by Y.
\[q_\ce{X}<0 \hspace{20px} \ce{and} \hspace{20px} q_\ce{Y}=−q_\ce{X}>0 \label{Eq4}\]
From the perspective of this two-object system, there was no net gain or loss of thermal energy, rather the available thermal energy was redistributed among the two objects. This spontaneous process resulted in a more uniform dispersal of energy.

Kielelezo:Wakati vitu\(\PageIndex{4}\) viwili katika joto tofauti vinawasiliana, joto hutoka kwa moto hadi kitu kilicho baridi.
Kama inavyoonyeshwa na michakato miwili iliyoelezwa, jambo muhimu katika kuamua upepo wa mchakato ni kiwango ambacho kinabadilisha kutawanyika au usambazaji wa suala na/au nishati. Katika kila kesi, mchakato wa hiari ulifanyika ambao ulisababisha usambazaji zaidi wa sare ya suala au nishati.
Mfano\(\PageIndex{1}\): Ugawaji wa Mambo wakati wa Mchakato wa Hifadhi
Eleza jinsi jambo linasambazwa tena wakati michakato yafuatayo ya hiari inafanyika:
- Sublimes imara.
- Gesi hupungua.
- Tone la rangi ya chakula limeongezwa kwenye glasi ya maji huunda suluhisho na rangi sare.
Suluhisho

Kielelezo\(\PageIndex{5}\) :( mikopo a: mabadiliko ya kazi na Jenny Downing; mikopo b: mabadiliko ya kazi na “Fuzzy Gerdes” /Flickr; mikopo c: mabadiliko ya kazi na Sahar Atwa)
- (a) Uwezeshaji ni uongofu wa imara (wiani wa juu) kwa gesi (wiani mdogo sana). Utaratibu huu hutoa usambazaji mkubwa wa suala hilo, kwani molekuli itachukua kiasi kikubwa zaidi baada ya mpito imara-kwa-gesi.
- (b) Uharibifu ni uongofu wa gesi (wiani mdogo) kwa kioevu (wiani mkubwa zaidi). Utaratibu huu hutoa usambazaji mdogo wa suala hilo, kwani molekuli itachukua kiasi kidogo baada ya mpito imara-kwa-gesi.
- (c) Mchakato katika swali ni dilution. Molekuli ya rangi ya chakula awali inachukua kiasi kidogo (tone la ufumbuzi wa rangi) kuliko wanavyofanya mara moja mchakato ukamilika (katika glasi kamili ya maji). Kwa hiyo mchakato unahusu usambazaji mkubwa wa jambo. Mchakato huo unaweza pia kutoa usambazaji wa sare zaidi wa suala, kwa kuwa hali ya awali ya mfumo inahusisha mikoa miwili ya viwango tofauti vya rangi (juu ya kushuka, sifuri katika maji), na hali ya mwisho ya mfumo ina mkusanyiko wa rangi moja.
Zoezi\(\PageIndex{1}\)
Eleza jinsi jambo na/au nishati inavyogawanywa tena wakati unapoweka canister ya hewa iliyosimamiwa ndani ya chumba.
Jibu:
Hii pia ni mchakato wa dilution, sawa na mfano (c). Inahusisha kuenea zaidi na zaidi ya sare ya suala kama hewa iliyosimamiwa katika canister inaruhusiwa kupanua ndani ya hewa ya chini ya shinikizo la chumba.
Muhtasari
Michakato ya kemikali na kimwili ina tabia ya asili ya kutokea katika mwelekeo mmoja chini ya hali fulani. Utaratibu wa pekee hutokea bila ya haja ya pembejeo ya nishati ya kuendelea kutoka kwa chanzo cha nje, wakati mchakato usio na kawaida unahitaji vile. Systems kufanyiwa mchakato hiari wanaweza au uzoefu faida au hasara ya nishati, lakini wao uzoefu mabadiliko katika njia jambo na/au nishati ni kusambazwa ndani ya mfumo.
faharasa
- mchakato usio na hiari
- mchakato ambayo inahitaji kuendelea pembejeo ya nishati kutoka chanzo cha nje
- mabadiliko ya hiari
- mchakato unaofanyika bila pembejeo ya kuendelea ya nishati kutoka chanzo cha nje


